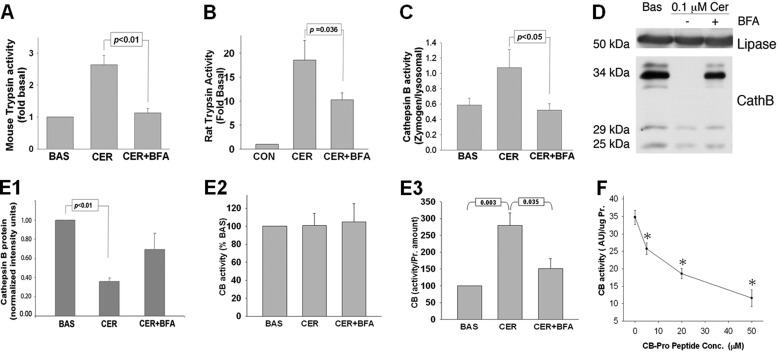
FIGURE 2.
BFA prevents trypsinogen activation and retains procathepsin B during treatment of pancreatic acini with 100 nm CER in vitro. A and B, trypsin activity assay in acinar cells after 30 min under CER stimulation demonstrates that BFA prevents CER-induced trypsinogen activation in both mouse (A) and rat acini (B). Data are presented as -fold change when compared with untreated acini (CON). C, cathepsin B activity assayed under conditions described in A and B shows that BFA reduces cathepsin B activation quantified as the ratio of the cathepsin B activity in the zymogen (1,300 × g) versus the lysosomal (12,000 × g) fraction. BAS, basal activity. D, WB of cathepsin B isoforms present in pancreatic acini shows that the 34-kDa procathepsin B (CathB) isoform is present in total cell lysate under basal condition and in acini pretreated with BFA followed by stimulation with CER but not in cells treated with CER alone. E1–E3, densitometry (E1) of the protein bands from WB shown in D demonstrates the reduction in the amount of active 25- and 29-kDa cathepsin B (CB) isoforms present in acini under CER-treated conditions, whereas the activity of cathepsin B in total cell lysates remains unchanged (E2). Histograms (E3) represent the quantification of the cathepsin B activity per the average protein content of the active, 25-kDa, and 29-kDa cathepsin B isoform under the same treatment condition as described in D from at least three independent experiments. The histograms show a dramatic increase in the cathepsin B activity/protein upon stimulation with CER, which is significantly inhibited by 50 μm BFA. F, quantification of the inhibitory effect of procathepsin B peptide on cathepsin B activity in acinar cell lysates. * indicates a significant (p < 0.05) decrease in activity at the corresponding peptide concentration compared to the activity in its absence.