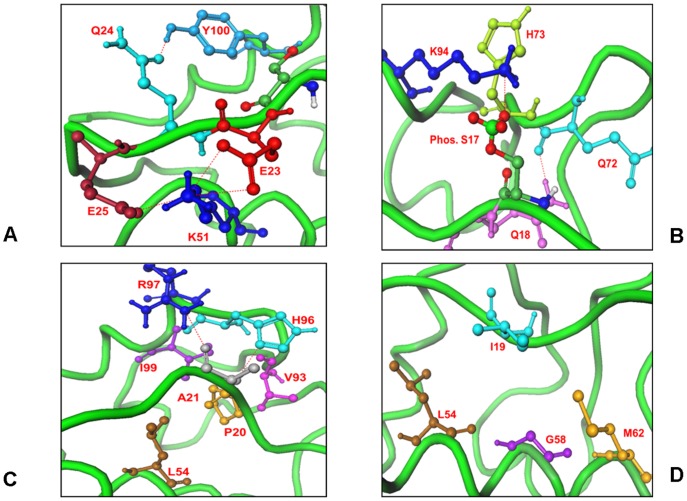
Figure 9. The Interaction Network of the Predicted Closed Form for the Phosphorylated pS17 Lid Interactions with MDM2.
(A) The “anchoring” interactions of the phosphorylated lid formed by E23, Q24 and E25 residues K51 and Y100 core domain residues. E23 and E25 are shown in red sticks, K51 in blue sticks, Q24 and Y100 in light blue sticks. (B) The hydrogen bonds formed by pS17 and Q18 with K94, Q72 and H73 residues. PS17 is shown in ball-stick-model with atom-based coloring, K94 in blue sticks. (C) These interactions were further supported by the favorable contacts of P20 occupying the first hydrophobic pocket and A21 backbone with the H96 and R97 residues. The hydrogen bonding network is supported by the packing interactions of the lid with the L54, H96 and Y100 residues. (D) The MDM2 residues L54, L57, G58, I61, M62, V93, H96 and I99 are involved in the hydrophobic contacts of both p53 and pS17 lid. I19 is not projected towards the binding cleft; because of it restricted movement due to P20.