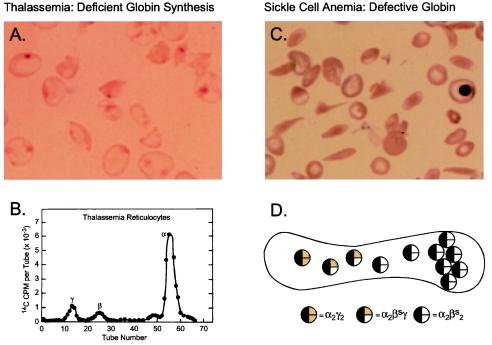
Figure 1.
Pathophysiology of hemoglobin disorders: Prospects for gene therapy. Normal human red cells contain 30 pg of hemoglobin, which in the adult is composed of two α and two β-globin chains (HbA = α2β2). The two globin chains are synthesized in nearly equal amounts in maturing erythroblasts. Before birth, γ-globin rather than β-globin is present in the hemoglobin tetramer. During the perinatal period, there is a switch from γ- to β-globin synthesis, resulting in the replacement of HbF with HbA in neonatal red blood cells. (A) Peripheral blood smear from a patient with severe homozygous β-thalassemia. The red blood cells are poorly hemoglobinized. The smear has been stained to depict the insoluble inclusions of α chains. (B) Biosynthesis of globin chains by erythroid cells from a patient with severe homozygous β-thalassemia. Incorporation of the radioactive amino acid into globin chains is characteristically abnormal with excess synthesis of α-globin and deficient synthesis of non-α- (γ- and β-) globin. Effective gene therapy could be achieved by increasing the synthesis of β-globin, the normal adult chain, or γ-globin, which is typically expressed during fetal life. (C) Blood cells from a patient with sickle cell anemia. The characteristic sickle shape reflects polymerization of hemoglobin, which occurs as a consequence of a single amino acid substitution, valine for glutamic acid, at position six on the surface of the hemoglobin molecule. (D) Expression of the naturally, anti-sickling fetal (γ-) globin in red cells from patients with sickle cell anemia reduces sickling propensity as a consequence of the formation of mixed tetramers (α2βsγ) in red blood cells. The mixed tetramers are unable to participate in polymerization, thereby effectively reducing the concentration of sickle hemoglobin (Hbα2βs2). Effective gene therapy could be achieved by introducing a functional γ-globin gene that expressed at least 20% the level of βs globin genes.