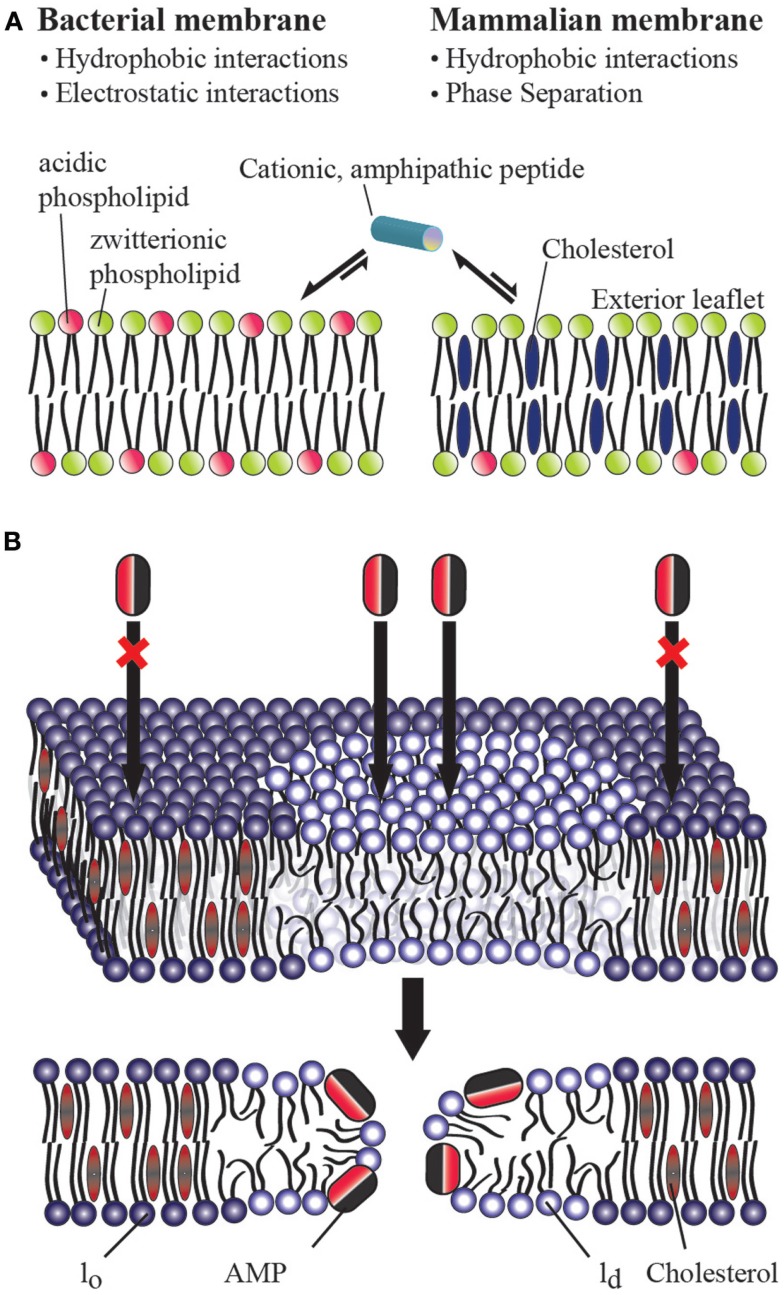
Figure 1.
(A) Role of cholesterol on the bacterial selectivity of antimicrobial peptides. Lipid bilayers mimicking bacterial (A) and eukaryotic (B) cell membranes are commonly used in in vitro studies on AMPs. In eukaryotic cell membranes, the outer leaflet consists primarily of zwitterionic phosphatidylcholine lipids (such as POPC), and cholesterol (∼25%) while the inner leaflet contains anionic lipids (such as POPS). Bacterial cell membranes typically lack cholesterol and contain ∼25% acidic lipids (like POPG and cardiolipin), and ∼55% phosphatidylethanolamine (POPE). AMPs have been shown to directly interact with the lipid bilayer of bacterial cell membranes and lyse the cell by disrupting the membrane via one of the several proposed mechanisms including barrel-stave, toroidal-pore, and detergent-type disturbances. The presence of cholesterol in the eukaryotic cell membrane enhances the rigidity of lipid bilayers to inhibit the membrane disruption activities of antimicrobial peptides. The electrostatic interaction between a cationic antimicrobial peptide and the anionic lipids (POPS) present in the outer leaflet of bacterial membranes plays a vital role in bacterial selectivity and the absence of cholesterol makes the membrane disruption by an AMP easier. In the case of Gram-negative bacteria, the presence of anionic lipopolysaccharides attracts cationic AMPs. (B) Mechanism of action of an antimicrobial peptide in a raft-containing membrane. In a heterogeneous mixture of lipids, the presence of cholesterol in the raft domain (lo) resists the permeation of an antimicrobial peptide while the disordered (ld) lipid domain is easily disrupted by an antimicrobial peptide.