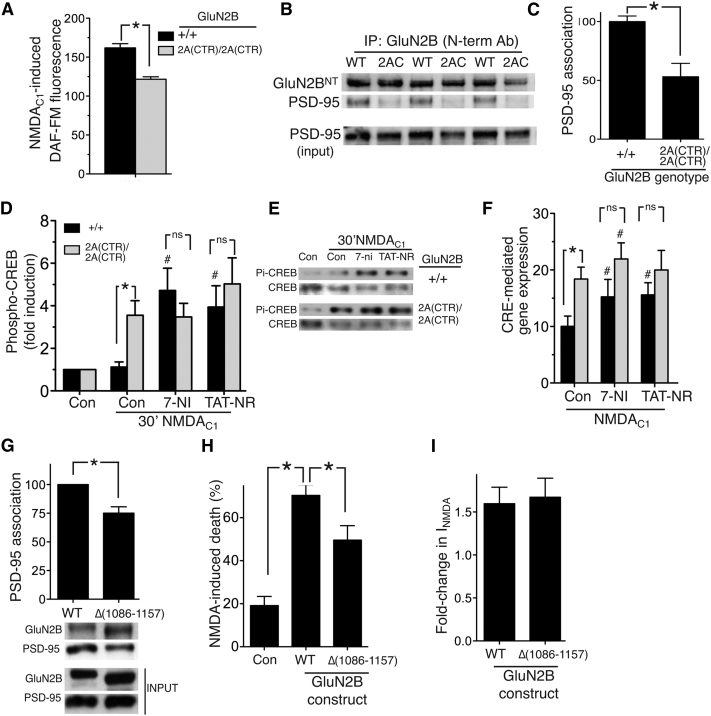
Figure 5.
The GluN2B CTD Couples More Strongly to a PSD-95-nNOS-Mediated CREB Shut-Off Pathway Than that of GluN2A
(A) DAF-FM-based NO assay (see Experimental Procedures) performed on neurons treated with NMDAC1 for 10 min. ∗p < 0.05; n = 6 (GluN2B+/+); n = 9 (GluN2B2A(CTR)/2A(CTR)). Mean ± SEM shown here and throughout the figure.
(B and C) GluN2BWT associates more strongly with PSD-95 than does GluN2B2A(CTR). GluN2B was immunoprecipitated from GluN2B+/+(WT) and GluN2B2A(CTR)/2A(CTR) (2AC) P7 cortical homogenates with a GluN2B N-terminal antibody. The presence of GluN2B and PSD-95 in the immunoprecipitate was analyzed by western blot, and the ratio of band intensities (PSD:GluN2B) was calculated (∗ p < 0.05; n = 11 (GluN2B+/+); n = 12 (GluN2B2A(CTR)/2A(CTR)).
(D and E) Western analysis of CREB phosphorylation (normalized to total CREB) in neurons pretreated as indicated with 7-nitroindazole (5 μM) or TAT-NR2B9c (2 μM) prior to NMDAC1 treatment for 5 or 30 min. ∗, p < 0.05; n = 10 (GluN2B+/+); n = 8 (GluN2B2A(CTR)/2A(CTR)). #, p < 0.05 t test comparison of the effect of the drug, compared to the (NMDA-treated) control.
(F) CRE reporter assay carried out as in Figure 4E. ∗p < 0.05; n = 5 (GluN2B+/+); n = 7 (GluN2B2A(CTR)/2A(CTR)). #, p < 0.05 paired t test comparison of the effect of the drug, compared to the control.
(G) Deletion of the GluN2B CTD between 1086–1157 lowers GluN2B affinity for PSD-95. HEK cells were transfected with plasmids encoding GluN1, PSD-95, and GluN2BWT or GluN2BΔ(1086–1157). After 24 hr, protein was extracted, and the association of GluN2B or GluN2BΔ(1086–1157) with PSD-95 was studied by coimmunoprecipitation, using an antibody to the N terminus of GluN2B. Upper, densitometric analysis of the resulting western blot (∗, p < 0.05 paired t test; n = 6). Lower, an example blot.
(H) Deletion of the GluN2B CTD between 1086–1157 lowers GluN2B-mediated excitotoxicity. Neurons were transfected with the indicated GluN2B constructs or β-globin (plus eGFP marker), and NMDA-induced death was assessed as described in Figure 1D (∗p < 0.05 paired t test [n = 8]; 250–300 cells analyzed per condition).
(I) Acute expression of GluN2BWT or GluN2BΔ(1086–1157) has a similar effect on NMDA-induced whole-cell currents. Neurons were transfected with the indicated constructs (plus eGFP marker), and whole-cell steady-state NMDAR-mediated currents evoked by 100 μM NMDA (normalized to cell capacitance) were compared to control-transfected neurons (β-globin; n = 4).
See also Figure S4.