Abstract
Background
The cross-sectional association between impaired glucose/diabetes and depression is inconsistent. We examined the longitudinal associations between diabetes, indicators of glucose metabolism and depressive symptoms over 2 years follow up.
Methods
Participants were 4338 men and women from the English Longitudinal Study of Ageing, a prospective study of community dwelling older adults (aged 62.9 ± 9.0 yrs, 45.2% men). Depressive symptoms were assessed at baseline and 2 years follow up using the 8 item CES-D scale. Haemoglobin A1C levels, fasting glucose and other biological and behavioural risk factors were also assessed at baseline.
Results
Approximately 11.5% of the sample were categorised with elevated depressive symptoms at follow up (a score ≥ 4 on the CES-D). There was an association between A1C and depressive symptoms at follow up (per unit increase, odds ratio [OR]= 1.17, 95% CI, 1.03 – 1.33) after adjustment for age and baseline CES-D. Cross-sectionally, the probability of depressive symptoms increased with increasing HbA1c levels until the value of 8.0% after which there was a plateau (p[curve]=0.03). Compared to those with normal fasting glucose, participants with diabetes (confirmed through self report or elevated fasting blood glucose) at baseline had elevated risk of depressive symptoms at follow up (OR=1.52, 95% CI, 1.01 – 2.30) after adjusting for depressive symptoms at baseline, behavioural and socio-demographic variables, adiposity and inflammation.
Conclusions
These data suggest that poor glucose metabolism and diabetes are risk factors for future depression in older adults. There was no evidence of a U-shaped association.
Keywords: diabetes, depressive symptoms, ageing, psychobiology, Haemoglobin A1C
Introduction
Depression and diabetes are both major public health concerns in the elderly population. For example, approximately 10% of adults in England aged 75 yrs and above have been diagnosed with diabetes (Shelton, 2008). Clinically significant levels of depression are also apparent in 11% – 25% of the general elderly population (Wancata et al. 2006). However, the determinants of mental health remain poorly understood.
The hypothesised association between diabetes and depression is theoretically feasible because depression could result from the biochemical changes directly caused by diabetes, its treatment, or from the distress associated with living with diabetes and its often debilitating consequences. For example, preliminary evidence found brain abnormalities, such as reduced white matter volume and enlarged cerebrospinal fluid space, in obese adolescents with type II diabetes, which might result from a combination of subtle vascular changes and glucose abnormalities (Yau et al. 2010). A common causal pathway for depression and diabetes is also a possibility, with early factors, such as low birth weight and childhood adversity predisposing individuals to both obesity/type 2 diabetes (de Lauzon-Guillain et al. 2010; Thomas et al. 2008) and depression (Colman et al. 2007). However, based on existing evidence the association between diabetes/glucose control and depression is contentious. Patients with diabetes tend to demonstrate a higher prevalence of depression than their diabetes-free counterparts (Ali et al. 2006, Mezuk et al. 2008), although the existing evidence is inconsistent with regards to the associations between glucose control and depression (Georgiades et al. 2007; Icks et al 2008; Adriaanse et al. 2008; Chida & Hamer 2008; Golden et al. 2008; Rhee et al. 2008; Holt et al. 2009; Kivimaki et al. 2009; Gale et al. 2010; Fisher et al. 2010). Some studies suggest a non-linear association between the two conditions. In the US Multi-Ethnic Study of Atherosclerosis, for example, individuals with impaired fasting glucose or undiagnosed diabetes had lower risk of incident depression than both non-diabetic individuals and patients with treated diabetes (Golden et al. 2008). A study from the British Whitehall II cohort reported greater levels of depression in participants with very high and very low fasting glucose (Kivimaki et al. 2009). These curvilinear associations were not, however, replicated in the Vietnam Experience study (Gale et al. 2010). Gaining a better insight into the association between diabetes and depression is therefore crucial as elevated depression risk at both low and high glucose levels would have important implications for prevention and treatment.
Previous studies have largely utilised a cross-sectional design. With the exception two prospective studies (Golden et al. 2008; Pan et al. 2010) that have indicated bi-directional associations between diabetes and depression, it is impossible to identify the direction of association from previous cross-sectional work. In order to simplify the interpretation of what is a potentially important relationship, we used data from the English Longitudinal Study of Ageing (ELSA), a prospective cohort study of older individuals. Previous analyses from ELSA have demonstrated an associated between depressive symptoms and incident diabetes (Demakakos et al. 2010). The aim of the present study was to examine the association of diabetes, levels of fasting glucose, and haemoglobin A1C at baseline, with new cases of elevated depressive symptoms arising over two years follow up. We used haemoglobin A1C because this biomarker has recently been highlighted as a gold standard indicator of diabetes risk (The International Expert Committee, 2009).
Methods
Study sample and procedures
ELSA is an ongoing cohort study that contains a nationally representative sample of the English population living in households (see ELSA user guide). The original ELSA cohort consists of men and women born on or before 29 February 1952. The sample was drawn from households that have participated in Health Survey for England (HSE) in 1998, 1999, and 2001. HSE recruits participants using multistage stratified probability sampling with postcode sectors selected at the first stage and household addresses selected at the second stage.
For the purposes of the present analyses data collected at wave 2 (2004-05) were used as the baseline, when clinical information was first gathered. Follow up data were collected two years later (2006-07). A total of 7666 participants attended the wave 2 (baseline) clinical assessment although 1230 did not attend the follow up, and a further 2098 of them were excluded because of missing biological data (n=1651) or incomplete data on other measures (n=447), leaving a final sample size of 4338 individuals (aged 62.9 ± 9.0 yrs, 45.2% men). Missing biological data was mainly because participants did not consent to give blood or were ineligible (participants with clotting and bleeding disorders, or taking anti-coagulant medication). In comparison with the overall sample, the sub-group used in the present analyses were slightly younger (62.9 vs. 63.8 yrs, p<0.001), from higher socioeconomic status groups (e.g., 35.4% vs. 27.8%, p<0.001, from managerial/professional level), had a lower prevalence of longstanding illness/disability (50.4% vs. 58.1%, p<0.001), and better health behaviours including lower rates of smoking (13.2% vs. 17.9%, p<0.001) and greater physical activity (32.6% vs. 23.4%, p<0.001, vigorously active ≥1/wk). In order to account for missing data all analyses were weighted for non-response, which is a standard procedure in order to account for survey non-response and unequal sample selection, thus providing more precise effect estimates (see ELSA User guide). Participants gave full informed consent to participate in the study and ethical approval was obtained from the London Multi-centre Research Ethics Committee.
Measurements
At baseline, data collection consisted of biological, psychosocial, demographic and health related information. Demographic and health-related questions included socioeconomic status as indexed by occupational social class (categorised as: managerial/professional, intermediate, semi routine/routine occupations), cigarette smoking (current or non-smoker), the frequency of participation in vigorous, moderate, and light physical activities (more than once per week, once per week, one to three times per month, hardly ever), frequency of alcohol intake (daily, 5-6/wk, 3-4/wk, 1-2/wk, 1-2/month, once every couple of months, 1-2/year, never) and presence of morbidity (including; doctor diagnosed heart disease, hypertension, diabetes, cancer, neuromuscular conditions, endocrine/metabolic conditions, epilepsy, bronchitis, asthma and other respiratory disorders, and complaints related to the stomach, digestive system, and bowel). Participants were categorised with diabetes if they reported a doctor’s diagnosis and/or use of diabetic medication. In addition, participants with diabetes were asked “Do you have sufficient knowledge to manage your diabetes?” and responses were stratified into two groups (“everything or most of what I need to know to manage my condition” and “some or a little of what I need to know”). Depressive symptoms were assessed at baseline and follow up using the 8-item Centre of Epidemiological Studies Depression (CES-D) scale. As in previous studies, we used a score of ≥4 to define cases of elevated depressive symptoms (Steffick 2000).
Nurses collected anthropometric data (weight, height) and blood samples. Blood samples were analysed for C-reactive protein (CRP), cholesterol, and A1C. In a sub-sample of participants we collected fasting blood samples for glucose. The analysis of the blood data was carried out at the Royal Victoria Infirmary (Newcastle-upon-Tyne, UK). Detailed information on the technicalities of the blood analysis, the internal quality control, and the external quality assessment for the laboratory have been described (Graig et al. 2006).
Statistical analyses
We calculated odds ratios (OR) and 95% confidence intervals (CI) for the risk of elevated depressive symptoms in relation to A1C using multiple logistic regression. These analyses were performed to examine both the cross-sectional and longitudinal associations. In multivariate models we adjusted for several covariates in a step-wise fashion; Model 1 contained basic variables including age, sex, baseline CES-D score; Model 2 contained behavioural and social covariates including social status, smoking, alcohol, physical activity; Model 3 contained clinical covariates including, self reported diabetes status, CRP, cholesterol, body mass index. This modelling strategy was devised a priori based on existing data linking these covariates with diabetes and mental health. In order to examine curvilinear associations we fitted an A1C squared term into the models and calculated the probability of depression by level of AIC based on these models to illustrate the shape of the association. In addition, participants were categorised into diabetes categories, which were used to model risk of depression. Diabetes categories were based on fasting glucose and self report; non-diabetic (fasting glucose <5.6 mmol/l and no self-reported diabetes), impaired fasting glucose (IFG: fasting glucose 5.6-6.9 mmol/l and no self-reported diabetes), and diabetes (fasting glucose ≥7.0 mmol/l, or self reported doctors diagnosis and/or use of diabetes medication). In addition, diabetes status was classified using A1C; non-diabetic (A1C <6%), impaired glucose tolerance (A1C = 6.0 – 6.5%), and diabetes (A1C ≥6.5% or self reported doctors diagnosis and/or use of diabetes medication). All analyses were conducted using SPSS version 14.
Results
Demographics
The proportion of the sample that was classified with elevated depressive symptoms (CES-D≥4) at baseline and follow up was 12.7% and 11.5%, respectively. Participants with depressive symptoms at follow up were older, more likely to be women, smoke, be physically inactive, non-drinkers, and come from lower socio-economic groups (Table 1). In addition, they were more likely to have a diabetes diagnosis and higher A1C, body mass index, and CRP.
Table 1.
Baseline characteristics of participants in relation to depressive symptoms at follow up.
| Variable, mean ± SD | Non-depressed (n=3840) |
Elevated depressive symptoms (n= 498) |
P-value |
|---|---|---|---|
| Age (yrs) | 62.8 ± 8.8 | 64.0 ± 9.9 | .004 |
| Men (%) | 47.1 | 30.9 | <.001 |
| Managerial/Professional (%) | 37.2 | 21.3 | <.001 |
| Current smokers (%) | 12.3 | 20.5 | <.001 |
| Physically inactive (%) | 15.1 | 29.9 | <.001 |
| Alcohol (% non-drinkers) | 7.7 | 17.7 | <.001 |
| Self reported diabetes (%) | 6.3 | 9.2 | .01 |
| A1C (%) | 5.5± 0.67 | 5.7± 0.83 | <0.001 |
| Body mass index (kg.m2) | 27.7± 4.6 | 28.3± 5.3 | .01 |
| Log C-reactive protein | 1.18± 0.72 | 1.40± 0.78 | <.001 |
| Cholesterol (mmol/L) | 5.95± 1.19 | 5.98± 1.24 | 0.58 |
Cross sectional associations between diabetes and depressive symptoms
In cross-sectional analyses, A1C (p=0.01) and [A1C]2 (p=0.03) were associated with depressive symptoms, suggesting the presence of a curvilinear association. Between the HbA1c range 4.5% to 9.0%, the probability of depressive symptoms increased with increasing HbA1c levels until the value of 8.0% after which there was a plateau (see Figure 1). The risk of depressive symptoms at baseline was elevated in participants that reported injecting insulin (n=70) (age adjusted odds ratio= 1.84, 95% CI, 1.02-3.34) and in diabetic participants who reported limited knowledge about diabetes management (n=44) (age adjusted odds ratio= 3.19, 95% CI, 1.48-6.89) compared to those with adequate knowledge.
Figure 1.
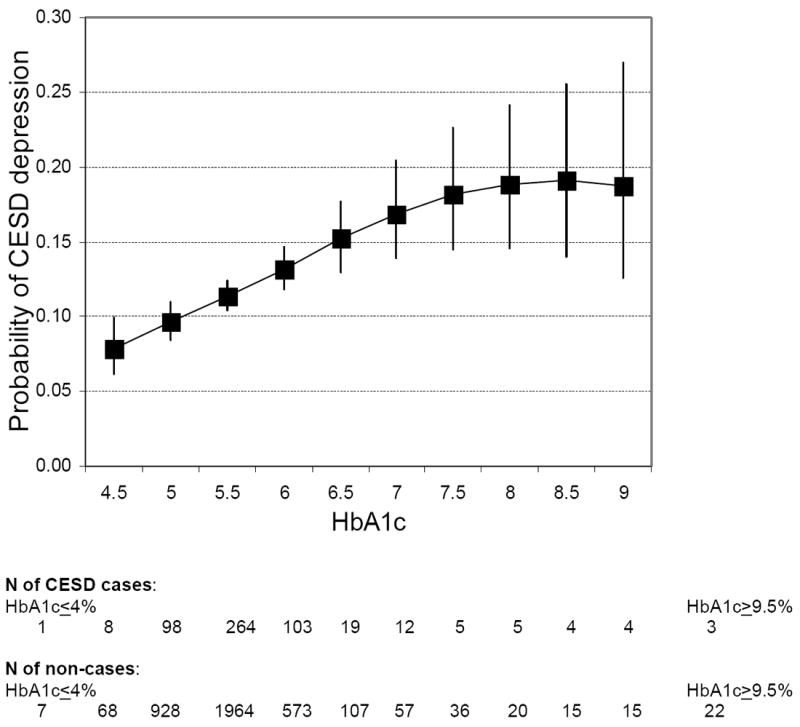
The association between glycated haemoglobin (HbA1C) and probability of elevated depressive symptoms (CES-D≥4) at baseline (N=4338, only HbA1C categories with n>10 shown).
Longitudinal associations between diabetes and depressive symptoms
In longitudinal analyses, A1C was associated with future risk of elevated depressive symptoms after controlling for depressive symptoms at baseline (see Table 2), although there was no evidence of a curvilinear association. The associations appeared to be slightly stronger in men but the sex-A1C interaction term was non-significant (p=0.08). The addition of further covariates to the model attenuated these associations, especially in women. In the fully adjusted model, the association between A1C and depressive symptoms no longer remained statistically significant at conventional levels. The removal of participants reporting depressive symptoms at baseline did not change the results. The other independent predictors of depressive symptoms in this sample included non-alcohol drinkers (odds ratio= 1.70, 95% CI, 1.17-2.46), physical inactivity (odds ratio= 1.33, 95% CI, 0.97-1.83), lower social status (odds ratio= 1.51, 95% CI, 1.15-2.00), and higher log CRP (odds ratio per unit increase= 1.18, 95% CI, 1.03-1.36).
Table 2.
Logistic regression models for HbA1c and risk of future depressive symptoms over 2 yrs follow up in ELSA (n=4338). Cases defined as a score ≥4 on the 8 item CES-D scale
| Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
|
|---|---|---|---|
| All (n=4338, cases=498) | |||
| A1C (per unit) | 1.17 (1.03 – 1.33) | 1.12 (0.98 – 1.28) | 1.08 (0.91 – 1.29) |
| p-value | 0.02 | 0.09 | 0.35 |
| Men (n=1961, cases=154) | |||
| A1C (per unit) | 1.23 (1.04 – 1.46) | 1.19 (0.99 – 1.44) | 1.20 (0.95 – 1.52) |
| p-value | 0.02 | 0.07 | 0.13 |
| Women (n=2377, cases=344) | |||
| A1C (per unit) | 1.14 (0.94 – 1.38) | 1.10 (0.90 – 1.33) | 0.96 (0.75 – 1.25) |
| p-value | 0.18 | 0.35 | 0.78 |
Model 1 adjusted for age and baseline CES-D score.
Model 2 plus smoking, alcohol intake, physical activity, social status
Model 3 plus C-reactive protein, cholesterol, body mass index, self reported diabetes.
When we examined the risk of depressive symptoms based on diabetes status (Tables 3a and b), participants with diabetes were at the highest risk of future depression, whether defined using fasting glucose or A1C. Impaired fasting glucose, as defined from fasting blood glucose levels, was not associated with future risk of depressive symptoms (Table 3a). However, impaired glucose tolerance, as defined from A1C was moderately associated with depressive symptoms (Table 3b). There was no statistically significant increased risk of depressive symptoms at follow up (age and baseline depression adjusted odds ratio= 1.20, 95% CI, 0.58-2.51) in participants that reported injecting insulin at baseline. Similarly, there was no association between knowledge about diabetes management and depressive symptoms at follow up (age and baseline depression adjusted odds ratio= 0.94, 95% CI, 0.34-2.58).
Table 3.
a. Association between baseline diabetes status (using fasting glucose)† and risk of future depressive symptoms (n= 2930)*
| Cases/N | Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
|
|---|---|---|---|---|
| Non-diabetic | 220/2244 | 1.00 | 1.00 | 1.00 |
| IFG | 35/385 | 0.86 (0.57 – 1.30) | 0.94 (0.62 – 1.43) | 0.92 (0.60 – 1.39) |
| Diabetic | 50/301 | 1.57 (1.07 – 2.29) | 1.55 (1.05 – 2.30) | 1.52 (1.01 – 2.30) |
| p-trend | 0.038 | 0.071 | 0.08 | |
|
| ||||
|
b. Association between baseline diabetes status (using glycated haemoglobin)‡ and risk of future depressive symptoms (n= 4338)
| ||||
| Non-diabetic | 397/3712 | 1.00 | 1.00 | 1.00 |
| IGT | 46/280 | 1.53 (1.05 – 2.24) | 1.39 (0.94 – 2.04) | 1.37 (0.93 – 2.02) |
| Diabetic | 55/346 | 1.46 (1.02 – 2.07) | 1.35 (0.94 – 1.92) | 1.36 (0.94 – 1.97) |
| p-trend | 0.016 | 0.087 | 0.105 | |
Model 1 adjusted for age and baseline CES-D score.
Model 2 adjusted as model 1 plus for sex, smoking, alcohol intake, physical activity, social status
Model 3 adjusted as model 2 plus for C-reactive protein, cholesterol, body mass index.
Diabetes categories based on fasting glucose and self report; non-diabetic (fasting glucose <5.6 mmol/l and no self-reported diabetes), impaired fasting glucose [IFG] (fasting glucose= 5.6-6.9 mmol/l), diabetic (based on either fasting glucose ≥7.0 mmol/l, self reported doctors diagnosis or use of diabetes medication).
data unavailable in1408 participants.
Diabetes categories based on A1C values and self report; non-diabetic (A1C <6 % and no self-reported diabetes), impaired glucose tolerance [IGT] (A1C= 6.0 – 6.5 % and no self-reported diabetes), diabetic (based on either A1C >6.5%, self reported doctors diagnosis or use of diabetes medication).
Discussion
In the present study we examined the longitudinal association between glucose metabolism and depressive symptoms in a large cohort of older British adults. We showed that A1C was associated with incident elevated depressive symptoms over 2 years follow up, especially in men.
Cross-sectionally, the probability of depressive symptoms increased with increasing HbA1c levels until approximately the value of 8% after which there was a plateau. In longitudinal analyses, we found a modest association between diagnosed diabetes at baseline and depressive symptoms at follow up. The magnitude of this association is comparable to a recent study that employed data from general practices to examine the association between diabetes and subsequent risk of depression (Aarts et al. 2009). Injecting insulin and limited knowledge about diabetes management were both associated with greater risk of reporting depressive symptoms at baseline, but these factors did not predict subsequent depression at follow up after taking into account the baseline association. Consistent with cross-sectional findings from other recent studies (Adriaanse et al. 2008; Gale et al. 2010), participants with impaired glucose metabolism at baseline were at risk of subsequent depression. However, in several other studies impaired glucose tolerance or undiagnosed diabetes was associated with lower risk of depression (Golden et al. 2008; Icks et al. 2008) or not associated with depression at all (Knol et al. 2007; Rhee et al. 2008; Holt et al. 2009; Aujla et al. 2009). Indeed, when we used fasting glucose as an indicator of impaired fasting glucose there was no association with depression. Therefore these discrepancies might possibly be explained by differences in the methods to assess glucose metabolism, and also characteristics of the samples and measures of depression. However, given that the majority of previous studies have been cross-sectional, the prospective nature of our study adds considerably to the current evidence base.
The highest probability of depression was observed at A1C levels of 8 – 9%, which might reflect unrecognised, pre-clinical diabetes and the presence of undiagnosed symptoms. We did not observe increased risk of depressive symptoms among individuals with very low glucose levels. Such an association was previously observed in the Whitehall II cohort (Kivimaki et al. 2009), but not in the Vietnam Experience Study, where study participants were, on average, 20 years younger (Gale et al. 2010). The reason for this discrepancy was suggested to be a higher prevalence of underlying chronic conditions that potentially relate to low glucose and increased depression risk in Whitehall II. However, the participants in present study were older than those in both previous studies and we observed no elevation in depression towards the low end of the A1C distribution. Lastly, slightly stronger associations between A1C and depressive symptoms were observed among men, although these sex differences were not statistically significant (p=0.08 for sex interaction). The reasons for this possible sex difference remain unclear and the findings are not consistent with a recent study in women (Pan et al. 2010). The mechanisms linking diabetes and depression also remain unclear. Depression could result from the biochemical changes directly caused by diabetes, its treatment, or from the distress associated with living with diabetes and its often debilitating consequences. For example, preliminary evidence found brain abnormalities, such as reduced white matter volume and enlarged cerebrospinal fluid space, in obese adolescents with type II diabetes, which might result from a combination of subtle vascular changes and glucose abnormalities (Yau et al. 2010). A common causal pathway for depression and diabetes is also a possibility, with early factors, such as low birth weight and childhood adversity predisposing individuals to both obesity/type 2 diabetes (de Lauzon-Guillain et al. 2010; Thomas et al. 2008) and depression (Colman et al. 2007).
The strengths of this study include the sampling of a large, representative general population-based group, and the well characterised study members which facilitates insights into the role of potential confounding factors, and the prospective element of the study design. The limitations of the present study should also be recognized. Participants retained in our analyses generally reported lower levels of depressive symptoms and better health compared with the overall sample, although the analytic approach that we used accounted for missing data in estimating the association between glucose indicators, diabetes and depression. In fact, weighting for non-response actually had a minimal impact on the results, providing evidence against bias due to selective sample retention. Although the definition of diabetes was not only based on self report but also on objective blood measures, we were unable to differentiate between type I and II. However, it is likely that the majority of cases were type II since this is by far the most prevalent condition in the general adult population.
Conclusions
These data from the English Longitudinal Study of Ageing suggests diabetes is associated with an excess risk of future depressive symptoms in older adults. Considering the totality of data from this study and previous investigations, there seems to be no convincing evidence to support elevated risk of depression at low levels of fasting glucose and A1C. Our findings support the current recommendations of the American Diabetes Association (2008) to screen diabetic patients for depression.
Acknowledgments
Funding sources
The data were made available through the UK Data Archive. The English Longitudinal Study of Ageing (ELSA) was developed by a team of researchers based at University College London, the Institute of Fiscal Studies and the National Centre for Social Research. The funding is provided by the National Institute on Aging in the United States (grants 2RO1AG7644-01A1 and 2RO1AG017644) and a consortium of UK government departments coordinated by the Office for National Statistics. MH is supported by the British Heart Foundation (RG 05/006); GDB is a Wellcome Trust Career Development Fellow (WBS U.1300.00.006.00012.01); MK is supported by the National Heart, Lung, and Blood Institute (R01HL036310-20A2) and the National Institute on Aging (R01AG034454-01), NIH, US and the Academy of Finland. The Medical Research Council (MRC) Social and Public Health Sciences Unit receives funding from the UK MRC and the Chief Scientist Office at the Scottish Government Health Directorates.
The funders had no role in the study design; in the collection, analysis and interpretation of data; in writing of the report; or in the decision to submit the paper for publication. The developers and funders of ELSA and the Archive do not bear any responsibility for the analyses or interpretations presented here.
Footnotes
Author contributions
MH had full access to the data, and takes responsibility for the integrity and accuracy of the results. All authors contributed to the concept and design of study, drafting and critical revision of the manuscript.
Conflict of interest
None of the authors have any competing interests to declare.
References
- Aarts S, van den Akker M, van Boxtel MP, Jolles J, Winkens B, Metsemakers JF. Diabetes mellitus type II as a risk factor for depression: a lower than expected risk in a general practice setting. European Journal Epidemiology. 2009;24:641–8. doi: 10.1007/s10654-009-9385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaanse MC, Dekker JM, Heine RJ, Snoek FJ, Beekman AJ, Stehouwer CD, et al. Symptoms of depression in people with impaired glucose metabolism or Type 2 diabetes mellitus: The Hoorn Study. Diabetic Medicine. 2008;25:843–9. doi: 10.1111/j.1464-5491.2008.02464.x. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabetic Medicine. 2006;23:1165–73. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- Aujla N, Abrams KR, Davies MJ, Taub N, Skinner TC, Khunti K. The prevalence of depression in white-European and South-asian people with impaired glucose regulation and screen-detected type 2 diabetes mellitus. PLoS One. 2009;4(11):e7755. doi: 10.1371/journal.pone.0007755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Hamer M. An association of adverse psychosocial factors with diabetes mellitus: a meta-analytic review of longitudinal cohort studies. Diabetologia. 2008;51:2168–78. doi: 10.1007/s00125-008-1154-1. [DOI] [PubMed] [Google Scholar]
- Colman I, Ploubidis GB, Wadsworth ME, Jones PB, Croudace TJ. A longitudinal typology of symptoms of depression and anxiety over the life course. Biological Psychiatry. 2007;62:1265–1271. doi: 10.1016/j.biopsych.2007.05.012. [DOI] [PubMed] [Google Scholar]
- de Lauzon-Guillain B, Balkau B, Charles MA, Romieu I, Boutron-Ruault MC, Clavel-Chapelon F. Birth weight, body silhouette over the life course, and incident diabetes in 91,453 middle-aged women from the French Etude Epidemiologique de Femmes de la Mutuelle Generale de l’Education Nationale (E3N) Cohort. Diabetes Care. 2010;33:298–303. doi: 10.2337/dc09-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demakakos P, Pierce MB, Hardy R. Depressive symptoms and risk of type 2 diabetes in a national sample of middle-aged and older adults: the English longitudinal study of aging. Diabetes Care. 2010;33:792–7. doi: 10.2337/dc09-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21/08/2008];ELSA user guide and documentation. UK Data Archive. http://www.data-archive.ac.uk/findingData/snDescription.asp?sn=5050.
- Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23–28. doi: 10.2337/dc09-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CR, Kivimaki M, Lawlor DA, Carroll D, Phillips AC, Batty GD. Fasting Glucose, Diagnosis of Type 2 Diabetes, and Depression: The Vietnam Experience Study. Biological Psychiatry. 2010;67:189–92. doi: 10.1016/j.biopsych.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Georgiades A, Zucker N, Friedman KE, Mosunic CJ, Applegate K, Lane JD, Feinglos MN, Surwit RS. Changes in depressive symptoms and glycemic control in diabetes mellitus. Psychosomatic Medicine. 2007;69:235–41. doi: 10.1097/PSY.0b013e318042588d. [DOI] [PubMed] [Google Scholar]
- Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299:2751–9. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graig R, Deverill C, Pickering K. Quality control of blood, saliva and urine analytes. In: Spronston K, Mindell J, editors. Health survey for England 2004, Methodology and documentation. Vol. 2. The Information Centre; London: 2006. pp. 34–41. [Google Scholar]
- Holt RI, Phillips DI, Jameson KA, Cooper C, Dennison EM, Peveler RC Hertfordshire Cohort Study Group. The relationship between depression and diabetes mellitus: findings from the Hertfordshire Cohort Study. Diabetic Medicine. 2009;26:641–8. doi: 10.1111/j.1464-5491.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- Icks A, Kruse J, Dragano N, Broecker-Preuss M, Slomiany U, Mann K, et al. Heinz Nixdorf Recall Study Investigator Group. Are symptoms of depression more common in diabetes? Results from the Heinz Nixdorf Recall study. Diabetic Medicine. 2008;25:1330–6. doi: 10.1111/j.1464-5491.2008.02585.x. [DOI] [PubMed] [Google Scholar]
- Kivimäki M, Tabak AG, Batty GD, Singh-Manoux A, Jokela M, Akbaraly TN, et al. Hyperglycemia, type 2 diabetes, and depressive symptoms: the British Whitehall II study. Diabetes Care. 2009;32:1867–9. doi: 10.2337/dc09-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol MJ, Heerdink ER, Egberts AC, Geerlings MI, Gorter KJ, Numans ME, Grobbee DE, Klungel OH, Burger H. Depressive symptoms in subjects with diagnosed and undiagnosed type 2 diabetes. Psychosomatic Medicine. 2007;69:300–5. doi: 10.1097/PSY.0b013e31805f48b9. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Manson JE, Willett WC, Ascherio A, Hu FB. Bidirectional association between depression and type 2 diabetes mellitus in women. Archives Internal Medicine. 2010;170:1884–91. doi: 10.1001/archinternmed.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee MK, Musselman D, Ziemer DC, Vaccarino V, Kolm P, Weintraub WS, Caudle JM, Varughese RM, Irving JM, Phillips LS. Unrecognized glucose intolerance is not associated with depression. Screening for Impaired Glucose Tolerance study 3 (SIGT 3) Diabetic Medicine. 2008;25:1361–5. doi: 10.1111/j.1464-5491.2008.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton N. Health Survey for England 2006. Diabetes. In: Craig R, Mindell J, editors. Cardiovascular disease and risk factors in adults. The Information Centre; London: 2008. pp. 63–84. [Google Scholar]
- Steffick DE. Documentation of affective functioning measures in the Health and Retirement Study (HRS/AHEAD Documentation. Report DR-005) Ann Arbor, MI: Survey Research Center, University of Michigan, US; 2000. Available at: http://hrsonline.isr.umich.edu/docs/userg/dr-005.pdf. [Google Scholar]
- The International Expert Committee. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care. 2009;32:1–8. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Hyppönen E, Power C. Obesity and type 2 diabetes risk in mid adult life: the role of childhood adversity. Pediatrics. 2008;121:e1240–1249. doi: 10.1542/peds.2007-2403. [DOI] [PubMed] [Google Scholar]
- Wancata J, Alexandrowicz R, Marquart B, Weiss M, Friedrich F. The criterion validity of the Geriatric Depression Scale: a systematic review. Acta Psychiatr Scand. 2006;114:398–410. doi: 10.1111/j.1600-0447.2006.00888.x. [DOI] [PubMed] [Google Scholar]
- Yau PL, Javier DC, Ryan CM, Tsui WH, Ardekani BA, Ten S, Convit A. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia. 2010;53:2298–306. doi: 10.1007/s00125-010-1857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


