Abstract
CK2 is a regulatory kinase implicated in embryonic development and in cancer. Among the CK2 substrates is β-catenin, a protein with dual function in Wnt signaling and cell adhesion. Previously, we reported that CK2 activity is required for β-catenin stability and we identified threonine (T) 393 as a major CK2 phosphorylation site in β-catenin. However, it is not known whether phosphorylation at T393 increases β-catenin stability and if so, what is the mechanism. In this study we investigate the molecular mechanism of β-catenin stabilization through phosphorylation at T393. We found that pseudophosphorylation of β-catenin at T393 resulted in a stable activated form of β-catenin with decreased affinity for Axin in vitro. This phosphomimetic mutant also displayed decreased regulation by Axin in vivo in a bioassay in Xenopus laevis embryos. In contrast, the binding of T393 pseudophosphorylated β-catenin to E-cadherin was unaffected. Further analysis showed that pseudophosphorylation at T393 did not prevent β-catenin phosphorylation by GSK3β. Interestingly, we found that in the presence of pseudophophorylated β-catenin and another activated form of β-catenin, the recruitment of GSK3β to Axin is enhanced. These findings indicate that phosphorylation of T393 by CK2 may affect the stability of β-catenin through decreased binding to Axin. In addition, the increased recruitment of GSK3β to the destruction complex in the presence of activated β-catenin mutants could be a feedback mechanism to suppress overactive Wnt signaling.
Keywords: CK2, β-catenin phosphorylation, β-catenin stability, Axin, E-cadherin, GSK3β, Wnt signaling
CK2 is a ubiquitously expressed, highly conserved, tetrameric serine-threonine kinase that regulates essential biological processes such as embryonic development [Xu et al., 1999; Buchou et al., 2003; Allada and Meissner, 2005; Lou et al., 2008], and has been implicated in tumorigenesis [Landesman-Bollag et al., 2001; Seldin et al., 2005]. CK2 has been involved in regulating cellular functions including cell growth, morphology and apoptosis [Guerra and Issinger, 1999; Litchfield, 2003; Canton and Litchfield, 2006; Ahmad et al., 2008]. Despite the emerging picture of the processes that CK2 is implicated in in vitro and in vivo, its precise function and mechanism of action is still unknown. In this regard, it has recently been shown that CK2 regulates canonical Wnt signaling. Thus, CK2 is necessary and sufficient for Wnt signaling [Dominguez et al., 2005], TCF transactivation and Wnt-target gene upregulation [Song et al., 2003; Hammerlein et al., 2005; Gao and Wang, 2006; Tapia et al., 2006; Wang and Jones, 2006]. Moreover, CK2 is transiently activated by Wnt [Gao and Wang, 2006] and among the CK2 substrates and interactors [Meggio and Pinna, 2003; Gyenis and Litchfield, 2008] is β-catenin [Song et al., 2000; Bek and Kemler, 2002], a key component of both canonical Wnt signaling and cell adhesion.
Canonical Wnt signaling is essential during embryonic development and for adult tissue function [Reya and Clevers, 2005]; aberrant activation of this pathway can lead to congenital diseases and cancer [Moon et al., 2004; Nusse, 2005]. Canonical Wnt signaling has been implicated in cell growth, stem cell maintenance and differentiation (for reviews on Wnt signaling see the Wnt home page at http://www.stanford.edu/~rnusse/wntwindow.html). The canonical pathway controls the expression of Wnt-responsive genes through stabilization of β-catenin, a transcriptional co-activator [Gordon and Nusse, 2006; Huang and He, 2008]. In the absence of Wnt signals, cytosolic β-catenin is destabilized through phosphorylation by casein kinase Iα (CKIα) and glycogen synthase kinase 3β (GSK3β) at its N-terminus within a “destruction” complex formed with the scaffold proteins Axin and adenomatous polyposis coli (APC). This phosphorylation marks β-catenin for proteasome-mediated proteolysis. Wnt binding to its receptor Frizzled (Fzl) and co-receptor low-density lipoprotein receptor-related protein (LRP) releases β-catenin from the “destruction” complex. As a consequence, β-catenin stability increases. However, the cascade of events that leads to β-catenin stabilization remains incompletely defined. Stabilized β-catenin translocates into the nucleus, where it binds DNA-binding factors of the lymphoid enhancer binding factor (LEF) and T-cell factor (TCF) family and activates downstream gene expression, including c-myc and cyclin D1 (for a list of target genes see the Wnt gene homepage).
In addition to its signaling role, β-catenin is important in cell adhesion at adherens junctions. β-catenin mediates cadherin-based cell-cell adhesion through interaction with α-catenin and the cytoplasmic region of cadherin [Gumbiner, 2000], which on its own, can interact with other proteins that participate in cell adhesion, like p120 [Provost and Rimm, 1999]. Binding to cadherin-adhesion complexes can increase β-catenin half-life [Heasman et al., 1994; Papkoff, 1997]. The adhesion and Wnt signaling roles of β-catenin appear to be linked. For example, E-cadherin binding prevents β-catenin nuclear localization and β-catenin/LEF-1-mediated transactivation [Orsulic et al., 1999; Gottardi et al., 2001], and overexpression of mutants and membrane targeted forms of β-catenin can increase β-catenin-specific transactivation through stabilization of endogenous cytosolic β-catenin [Miller and Moon, 1997; Hagen et al., 2004; Somorjai and Martinez-Arias, 2008], although not in all instances [Cox et al., 1999].
Recently, the serine/threonine kinase CK2 has been proposed to interact with and to phosphorylate β-catenin in vitro [Song et al., 2000; Bek and Kemler, 2002]. Two different CK2 phosphorylation regions have been identified on β-catenin. Bek and Kemler [2002] identified the N-terminal residues S29, T102 and T112 as CK2 phosphorylation sites in vitro. Biochemical studies using a β-catenin mutant with alanines in these sites suggested that CK2 phosphorylation of those residues may increase the binding between β-catenin and α-catenin and may promote β-catenin degradation. This last hypothesis was supported by the fact that CK2 was shown to act synergistically with GSK3β in phosphorylating β-catenin in vitro, and the β-catenin alanine mutant was shown to have increased stability through diminished Axin binding [Bek and Kemler, 2002]. In contrast, we have shown that the activity of CK2 is necessary and sufficient for β-catenin stability and function in epithelial cell lines and Xenopus laevis embryos [Song et al., 2000, 2003; Dominguez et al., 2004]. We identified T393 as a major CK2 phosphorylation site on β-catenin [Song et al., 2000, 2003], and showed that an unphosphorylatable mutant, 393A β-catenin, has diminished stability [Song et al., 2003]. However, it has not yet been determined whether phosphorylation at T393 is sufficient to increase β-catenin stability, and whether T393 regulates β-catenin stability by affecting the interaction with Wnt signaling components or with cadherin complexes.
Here, we show that phosphorylation of β-catenin at T393 is sufficient to generate an active form of β-catenin with increased stability correlating with increased Wnt-target gene expression. For this we use a pseudophosphorylated or phosphomimetic mutant of β-catenin with an aspartic acid substitution at position T393. We show that the mechanism of stabilization of CK2-pseudophosphorylated β-catenin is through decreased regulation by Axin but not through cadherin binding. We show that CK2-pseudophosphorylation of β-catenin did not prevent its phosphorylation by GSK3β. Interestingly, our data indicate that stable forms of β-catenin, including β-catenin phosphorylated at T393, can induce the recruitment of GSK3β into the destruction complex, potentially as a feedback mechanism to suppress overactive Wnt signaling.
MATERIALS AND METHODS
PLASMIDS AND REAGENTS
In order to obtain myc-tagged versions of human β-catenin in a eukaryotic expression vector, β-catenin (either wildtype, 393A, or 393D) was excised from pGET-5X-1-β-catenin plasmids [Song et al., 2003] by digestion with BamHI and SmaI and was subcloned into p13M, 3′ of the myc sequence. From, pJ13M-myc-β-catenin wildtype (WT), myc-β-catenin (WT) was subcloned into pCS2 by PCR with primers flanked by the ClaI restriction site and sequenced. A SacII fragment of myc-β-catenin (393A, 393D) containing the last 529 amino acids was subcloned onto a backbone of pCS2-β-myc-β-catenin T393A where the SacII fragment was previously excised, to obtain pCS2-myc-β-catenin T393A and pCS2-myc-β-catenin T393D. pCS2-myc-β-catenin constructs were sequenced to ensure subcloning of the proper mutant.
Other plasmids utilized in this study are: pCS2-HA-XAxin (gift from Dr. Ramesh A. Shivdasani), pBAT-myc-ΔN-β-catenin (gift from Dr. Walter Birchmeier), pCS2-FLAG-β-catenin (S33/37A, T41A, S45A) (gift from Dr. Xi He) and pCDNA3-hE-cadherin (gift from Dr. Barry M. Gumbiner).
EMBRYO MANIPULATION AND MICROINJECTION
In vitro fertilization, embryo culture, injection and visualization were carried out as described [Newport and Kirschner, 1982; Dominguez et al., 2004]. The myc-β-catenin (393A or 393D mutant) and HA-XAxin mRNAs were in vitro transcribed (mMessage mMachine™, Ambion), quantified with a standard RNA marker (New England Biolabs) in a denaturing agarose gel, and injected equatorially into two ventral blastomeres at stage 2 or 3. Embryonic development was analyzed at gastrula (stage 10.5) and tadpole (stage 38) stages [Nieuwkoop and Faber, 1967]. Embryos were fixed at stage 38 as described [Dominguez et al., 2004]. For categorizing axis defects, tadpoles were assessed according to the dorsoanterior index (DAI; [Kao and Elinson, 1988]).
CELL CULTURE, TRANSFECTION, AND TREATMENT
C57MG cells and HEK293T cells were grown in DMEM (Mediatech Inc.) supplemented with 10% FBS (Atlanta Biologicals), 4 mM l-glutamine, 50 U/ml penicillin, 50 mg/ml streptomycin (Mediatech Inc.) in a 5% CO2 incubator at 37°C. Transfections were performed using Dreamfect (for C57MG cells, OZ Biosciences) or Lipofectamine 2000 (for HEK293T cells and C57MG cells, Invitrogen) according to the manufacturer’s instructions. Total DNA was balanced with the addition of empty vector. A plasmid encoding GFP (pEFGP) was included as a control for transfection efficiency. For steady-state protein quantity analysis, C57MG cells were harvested 24 h after transfection. For serum starvation, C57MG cells were incubated for 2.5 h in DMEM supplemented with 0.1% FBS and 4 mM l-glutamine 24 h after transfection and harvested at the times indicated in Results Section. For cycloheximide (CHX, Sigma) treatment, cells were treated with 50 µg/ml CHX 24 h after transfection and cells were harvested at different times for immunoblot analysis.
QUANTITATIVE IMMUNOBLOT ANALYSIS
Cells were harvested by scraping after 2 washes in ice-cold PBS and were lysed in buffer A (50 mM Tris–HCl/pH 8.0, 125 mM NaCl, 1% NP-40, 10 mM NaF, 10 mM PMSF, 10 mM Na3VO4, l0 mM NaPPi, 5 µg/ml leupeptin, aprotinin, antipain and pepstatin). Protein concentration was determined by BCA assay (Pierce). Protein lysates were boiled in Laemmli buffer (Boston Bioproducts) and proteins separated by SDS–PAGE were electroblotted onto PVDF membranes (Millipore). The membranes blocked with 10% goat serum (Sigma) in PBS-Tween (PBS-T), were incubated for 90 min with anti-c-myc (9E10, Roche), anti-E-cadherin (BD Biosciences), anti-HA (Covance), anti-p120 and anti-β-catenin (BD Biosciences), anti-β-tubulin (Sigma), anti-GSK3β (BD Biosciences) or antiphospho-β-catenin (Ser33/37/Thr41) (Cell Signaling) antibodies followed by incubation in HRP-conjugated secondary IgG antibodies (Jackson ImmunoResearch) for 1 h. Peroxidase activity was visualized by ECL (GE Healthcare). Fluor-S (Biorad) was used to quantify band intensities from exposed film or from PVDF membranes. Incubation times, antibody concentrations, protein loading, and densitometry were optimized to be within the linear range of the assay.
IMMUNOPRECIPITATION
HEK293T cells were transfected with the indicated plasmids and 24 h later, cells were incubated for 4 h in medium containing 0.5% FBS and lysed in buffer A as described above. Cleared lysate (220–300 µg protein) was incubated with 1.5 µg of anti-c-myc (9E10), anti-HA, anti-FLAG or anti-E-cadherin for 2 h at 4°C. The antibody-lysate mixture was cleared at 20,000g for 10 min and supernatant was incubated with 8 µl of protein G-sepharose beads (GE Healthcare) for 30 min at 4°C. The beads were pelleted at 900g for 2 min, washed 3 times for 5 min with washing buffer (50 mM Tris, pH 8.0, 50 mM NaCl, 0.1% NP40), boiled in Laemmli buffer and subjected to SDS–PAGE for immunoblot analysis.
RNA ISOLATION AND RT-QUANTITATIVE PCR
Real-time PCR reactions were performed as described [Currier et al., 2005]. Briefly, total RNA from transfected C57MG cells was isolated with Trizol according to manufacturer’s instructions (Invitrogen) and its concentration was determined by spectrometry (BioRad). First-strand cDNA was synthesized using 2 µg of total RNA (DNase-treated) in a 50 µl reverse transcriptase (RT) reaction mixture (Invitrogen) with and without RT. Real-time PCR reactions were performed in a 20–25 µl mixture containing 1/20 volume of cDNA preparation, 2× TaqMan Universal PCR Master Mix (Applied Biosystems) and 1–1.25 µl Assay on Demand Gene Expression Reagent for c-myc or β-glucuronidase (GUS) as a loading control (Applied Biosystems). Real-time quantitations were performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems). The reaction mixture was initially denatured for 10 min at 95°C, followed by 40 cycles of denaturation at 95°C for 15 s and annealing/extending at 60°C for 1 min. Background signal was eliminated and Ct values were determined using the Software Design Specification (SDS) version 1.1 analysis software (Applied Biosystems). Reactions were performed within the linear range for GUS and c-myc.
STATISTICS
The Student’s t-test was used to compare the transactivation ability, steady-state levels and N-terminus phosphorylation status of 393D and 393A myc-β-catenin. The Fisher exact test was used to compare the effect of HA-XAxin on the ectopic axis induction ability of 393D and 393A myc-β-catenin. For this, embryos were categorized into five groups: DAI < 5, single axis, partial ectopic axis, complete ectopic axis, and gastrulation defect. The numbers of embryos in each category were counted and the Fisher exact test was applied.
RESULTS
393D PHOSPHOMIMETIC MUTANT β-CATENIN HAS INCREASED STABILITY
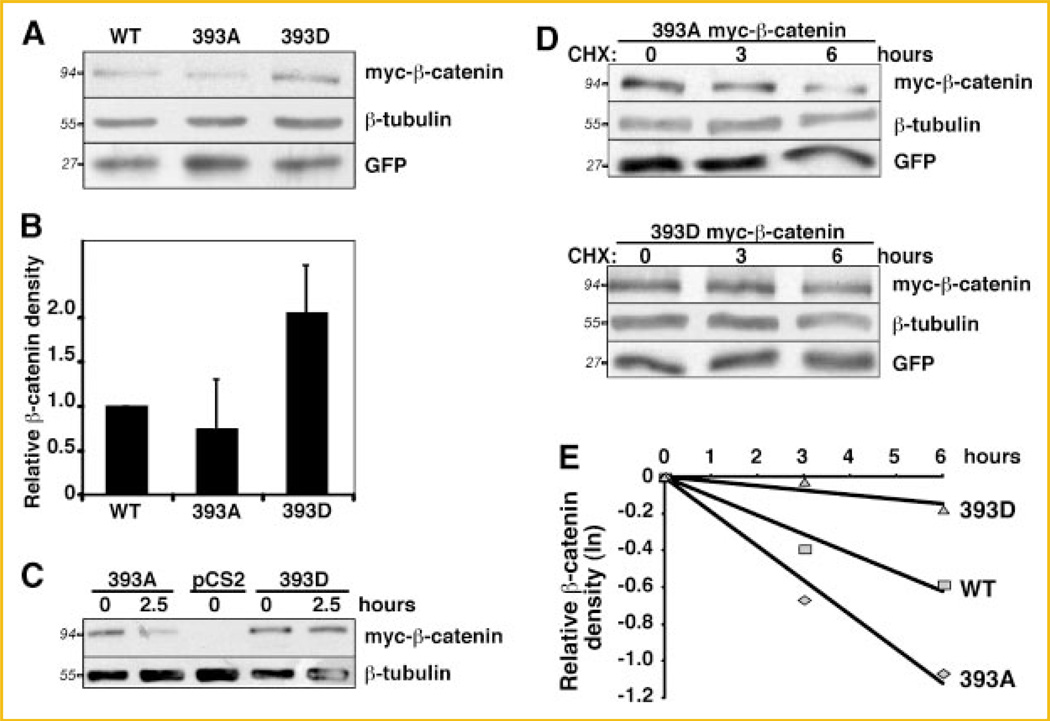
To test whether phosphorylation of β-catenin at T393 could result in increased β-stability, a mutant β-catenin with a mimetic of a phosphate group and a mutant that cannot be phophorylated were used. To mimic the effect of an acidic phosphate group at T393, the threonine was substituted with an aspartic acid (393D). The non-phosphorylatable mutant was obtained by substituting the threonine with an alanine (393A). To determine whether phosphorylation at T393 may promote increased stability of β-catenin, we compared the protein levels of myc-tagged 393D and 393A β-catenin in C57MG cells. C57MG cells, an immortalized non-transformed murine breast epithelial cell line [Vaidya et al., 1978], was chosen for these experiments, as canonical Wnt signaling is activated in response to Wnts [Giarre et al., 1998; Shibamoto et al., 1998] and activating mutations of β-catenin [Staal et al., 1999]. C57MG cells also have a functional proteasome degradation pathway [Aberle et al., 1997] and had been extensibly used for β-catenin stability studies [Song et al., 2000, 2003]. Our data showed that steady-state levels of 393D protein are two times higher than 393A levels (Fig. 1A,B). β-Tubulin was used as a loading control and a GFP expressing plasmid was used as a control for transfection efficiency. The difference in protein levels between these β-catenin mutants was increased in C57MG cells incubated in low serum conditions (Fig. 1C).
Fig. 1.
393D β-catenin is more stable than 393A β-catenin. A: C57MG cells were lysed 24 h after transfection with 393A, 393D and wildtype myc-β-catenin plasmids and processed for immunobloting with the indicated antibodies. One representative experiment of four is shown. B: Histogram representing normalized mean ± SD from four independent experiments as in (A). The myc-β-catenin band intensity was corrected for loading (β-tubulin) and transfection efficiency (GFP). 393D was normalized to 1. C: C57MG cells were transfected with 393A, 393D myc-β-catenin plasmids, and empty vector (pCS2). Twenty-four hours after transfection cells were incubated in media containing 0.1% FBS and harvested after 0 or 2.5 h. One representative experiment out of three is shown. D: Half-life analysis: 24 h after transfection with 393A, 393D or wildtype (not shown) myc-β-catenin, C57MG cells were treated with 50 µg/ml CHX and lysed at indicated times (in hours), lysates were subjected to immunoblot. E: Logarithmic plot of myc-β-catenin degradation over time. The myc-β-catenin band intensity from two independent experiments was corrected for loading (β-tubulin) and transfection efficiency (GFP), and normalized to the zero time point. The half-life was calculated according to the slope of the lines. Apparent protein molecular weights are shown in italics.
In C57MG cells treated with cycloheximide (CHX), the 393D β-catenin displayed a significantly longer half-life (Fig. 1D,E). β-tubulin was used as a loading control and a GFP expressing plasmid was used to assess transfection efficiency. The calculated average from two independent experiments showed that the half-life of phosphomimetic myc-β-catenin (40.7 h) was eight times higher than that of 393A myc-β-catenin (5.4 h). The half-life of the wildtype protein was intermediate (9.6 h) suggesting that wildtype β-catenin may exist in both phosphorylated and un-phosphorylated states (Fig. 1E). This is not unexpected as both β-catenin and CK2 are regulated by growth factors present in serum [Bosc et al., 1999; Chen et al., 2000; Olmeda et al., 2003; Homma and Homma, 2008].
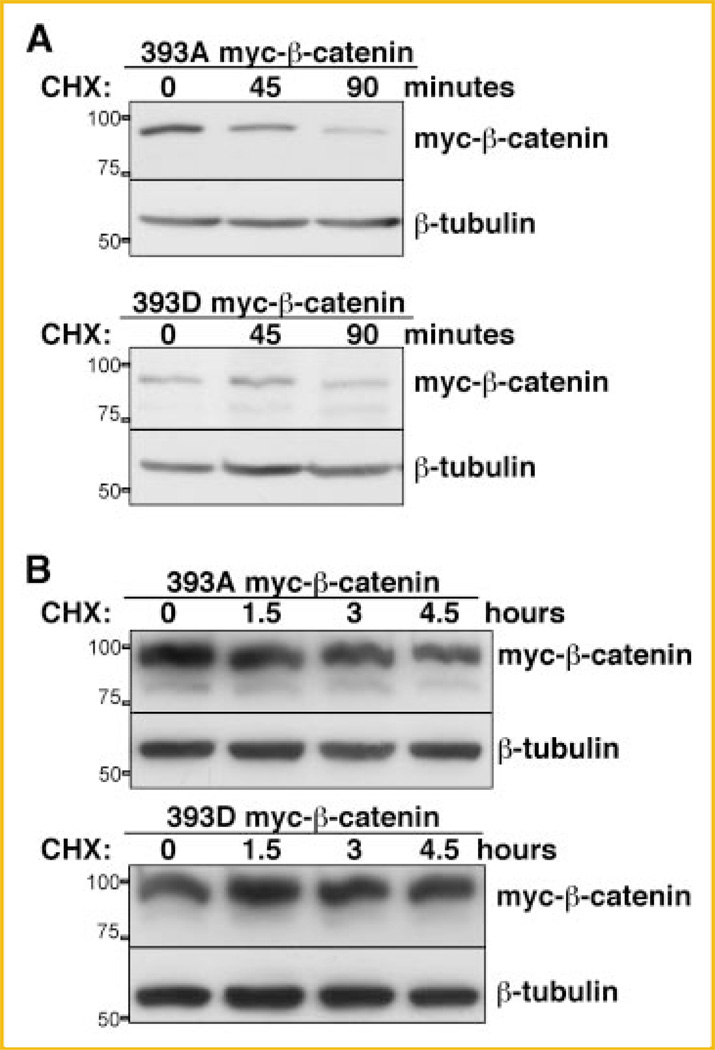
We extended these observations to two additional cell lines, HEK293T, a human embryonic kidney cell line, and L, a C3H mouse fibroblast, that, like C57MG, respond to Wnt activation by upregulating endogenous β-catenin [Shibamoto et al., 1998; Julius et al., 1999]. The increased stability of 393D myc-β-catenin was also observed in these cell lines upon CHX treatment (Fig. 2A,B). All together, these data indicate that phosphorylation of β-catenin at T393 is sufficient to stabilize β-catenin.
Fig. 2.
CK2 pseudophosphorylated β-catenin has increased half-life in L cells and HEK293T. A: Twenty-four hours after transfection with 393D or 393A myc-β-catenin, L cells were trypsinized, resuspended in DMEM and cultured in suspension in 50 ml Falcon tubes (BD) and incubated in 37°C water bath. Cells were treated with 50 µg/ml CHX. One ml of cell suspension was taken at different time points, lysed directly in Laemmli buffer, and separated onto SDS–PAGE followed by immunoblot analysis with anti-c-myc and anti-tubulin antibody. B: Twenty-four hours after transfection with 393D or 393A myc-β-catenin, HEK293T cells were trypsinized and split into a 6-well plate. Fifty µg/ml CHX was added to the cells after replating. Cells were lysed at different time points for immunoblot analysis with anti-c-myc antibody to detect myc-β-catenin. Position and weight of the molecular markers utilized is indicated on the left of the immunoblots.
ENHANCED TRANSACTIVATION OF 393D PHOSPHOMIMETIC β-CATENIN
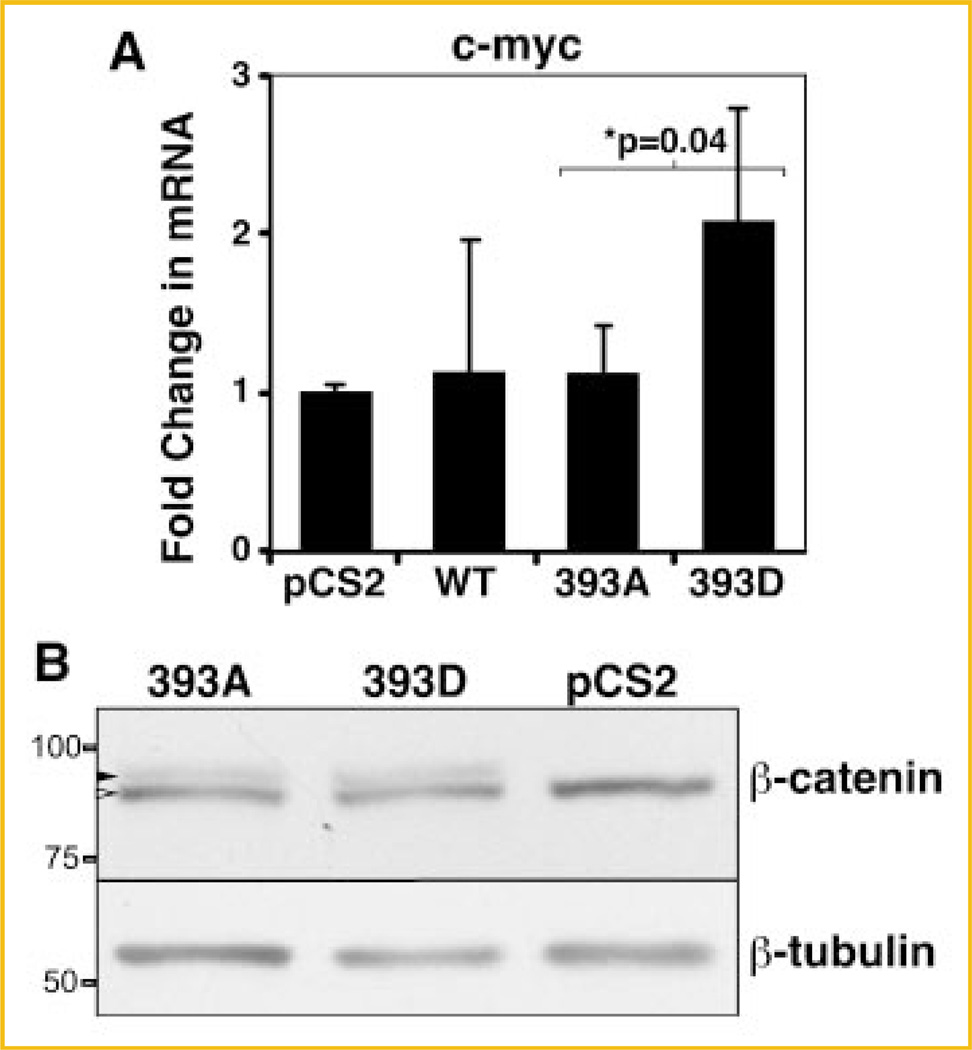
To determine whether the increased stability of 393D β-catenin is functionally significant, we evaluated the expression of a Wnt target gene, c-myc. Expression of endogenous c-myc was evaluated in three independent experiments in C57MG cells expressing β-catenin mutants. RT-qPCR analysis of c-myc in transfected C57MG cells showed that pseudophosphorylation of T393 lead to enhanced c-myc expression. The level of c-myc mRNA was twofold greater (P = 0.04) in 393D-myc-β-catenin than in 393A myc-β-catenin transfected cells (Fig. 3A).
Fig. 3.
T393 pseudophosphorylated β-catenin is transcriptionally more active than 393A. A: mRNA was isolated from pCS2 (control plasmid), WT, 393A or 393D myc-β-catenin transfected C57MG cells and subjected to RT-qPCR. Results are expressed as mRNA levels normalized to the control β-glucuronidase (GUS). Data represent mean ± SD is from four independent experiments. Asterisk (*) indicates P < 0.05. B: Endogenous β-catenin levels are not affected by mutant β-catenin transfection. C57MG cells were lysed 24 h after transfection with 393D or 393A myc-β-catenin or pCS2. Cell lysates were subjected to immunoblotting with the indicated antibodies. Black arrowhead represents myc-β-catenin, and white arrowhead points to endogenous β-catenin. Position and weight of the molecular markers utilized is indicated on the left of the immunoblots.
In different models, exogenous expression of β-catenin causes stabilization of endogenous cytosolic β-catenin, which leads to increase in β-catenin-specific gene transactivation [Miller and Moon, 1997; Hagen et al., 2004; Somorjai and Martinez-Arias, 2008]. We therefore tested whether the levels of endogenous β-catenin increased upon expression of 393D-myc-β-catenin. We found that expression of similar levels of 393D-myc-β-catenin or 393A myc-β-catenin in C57MG cells had no effect on the levels of endogenous β-catenin (Fig. 3B). Taken together, these results suggest that phosphorylation of β-catenin at T393 generates a stabilized form of β-catenin with increased transactivation potential. Based on the literature, to determine how T393-pseudophosphorylated β-catenin acquired enhanced stability and transactivation potential, two potential mechanisms were examined: decreased binding to the destruction complex component, Axin, or changes in cadherin binding. We tested these possibilities below.
T393 MUTANTS OF β-CATENIN HAVE NO EFFECT ON E-CADHERIN BINDING
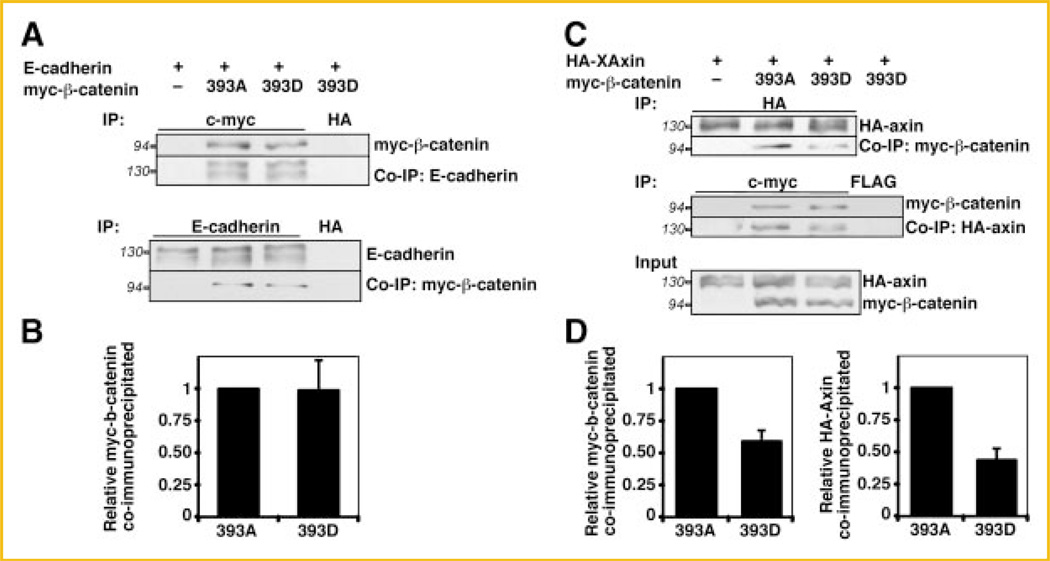
We first tested whether pseudophosphorylation of β-catenin at T393 altered affinity for E-cadherin compared to unphosphorylated β-catenin. In these experiments we did not test wildtype β-catenin as we cannot control its phosphorylation status when expressed in cells. To perform this experiment we utilized HEK293T cells, as the transfection efficiency was higher than that of C57MG or L cells. HEK293T cells were transfected with E-cadherin, and with 393D or 393A myc-β-catenin. Anti-c-myc or anti-E-cadherin antibodies were used for immunoprecipitation (IP) of the lysates (Fig. 4A). Quantitation of three independent experiments showed that similar amount of 393D myc-β-catenin and 393A myc-β-catenin co-immunoprecipitated with E-cadherin (Fig. 4B). As a control, p120, a protein associated with membrane-bound cadherin [Provost and Rimm, 1999] also co-immunoprecipitated in equal amounts (not shown). These results suggest that, as opposed to the N-terminal CK2 phosphorylation sites, phosphorylation at T393 does not regulate binding to cadherin complex components. Thus, the increased stability of 393D β-catenin cannot be explained by changes in E-cadherin binding.
Fig. 4.
T393 pseudophosphorylation of β-catenin affects Axin binding but not E-cadherin binding. A: HEK293T cells were transfected with 393A and 393D myc-β-catenin either alone or with E-cadherin. Twenty-four hours after transfection, cells were starved in medium containing 0.5% FBS for 4 h. Anti-c-myc (upper panel) and anti-E-cadherin (lower panel) were used for immunoprecipitation from the cell extracts from the transfected cells. Anti-HA antibody was used for mock IP. B: Histogram representing quantitation from three experiments shows that E-cadherin bind to 393D and 393A myc-β-catenin with equal affinity. C: HEK293T cells were transfected with 393A or 393D myc-β-catenin either alone or with HA-XAxin. Twenty-four hours after transfection, cells were starved in medium containing 0.5% FBS. Anti-HA (upper panel) and anti-c-myc (middle panel) antibodies were used for immunoprecipitations from the cell extracts; anti-FLAG antibody was used for mock IP. This was followed by immunoblot with indicated antibodies. Expression levels of exogenous proteins are shown in the lower panel (input). D: Histograms representing quantitation from three independent experiments: (left) relative myc-β-catenin coimmunoprecipitated by HA-XAxin (the ratio between 393A myc-β-catenin and HA-XAxin was normalized to 1), and (right) relative HA-XAxin coimmunoprecipitated by myc-β-catenin (the ratio between HA-XAxin and 393A myc-β-catenin was normalized to 1). Apparent protein molecular weights are shown in italics.
REDUCED BINDING OF 393D β-CATENIN TO AXIN
We next examined whether the increased stability of the 393D β-catenin mutant was due to decreased binding to the destruction complex. If so, the 393D myc-β-catenin mutant should have diminished affinity for Axin compared to the 393A β-catenin mutant. To test this hypothesis, HEK293T cells were transfected with 393D or 393A myc-β-catenin, with HA-XAxin. Anti-HA or anti-c-myc antibodies were used for IP of the lysates. When equal amounts of HA-XAxin were immunoprecipitated, more 393A myc-β-catenin was co-immunoprecipitated compared to the 393D mutant (Fig. 4C, upper panel). A similar result was observed in the reverse IP (Fig. 4C, middle panel). Exogenous proteins were expressed at similar levels (Fig. 4C, input). Quantitation of three independent experiments shows that the binding of HA-XAxin to 393D myc-β-catenin was 50% and 60% of that of 393A myc-β-catenin, in the direct or reverse IPs, respectively (Fig. 4D). These data indicate that phosphorylation of β-catenin on T393 lowers its affinity for Axin and makes β-catenin less accessible to the destruction complex, providing a mechanism for the increased stability of 393 phosphomimetic β-catenin.
REDUCED REGULATION OF 393D β-CATENIN BY AXIN IN VIVO IN X. LAEVIS EMBRYOS
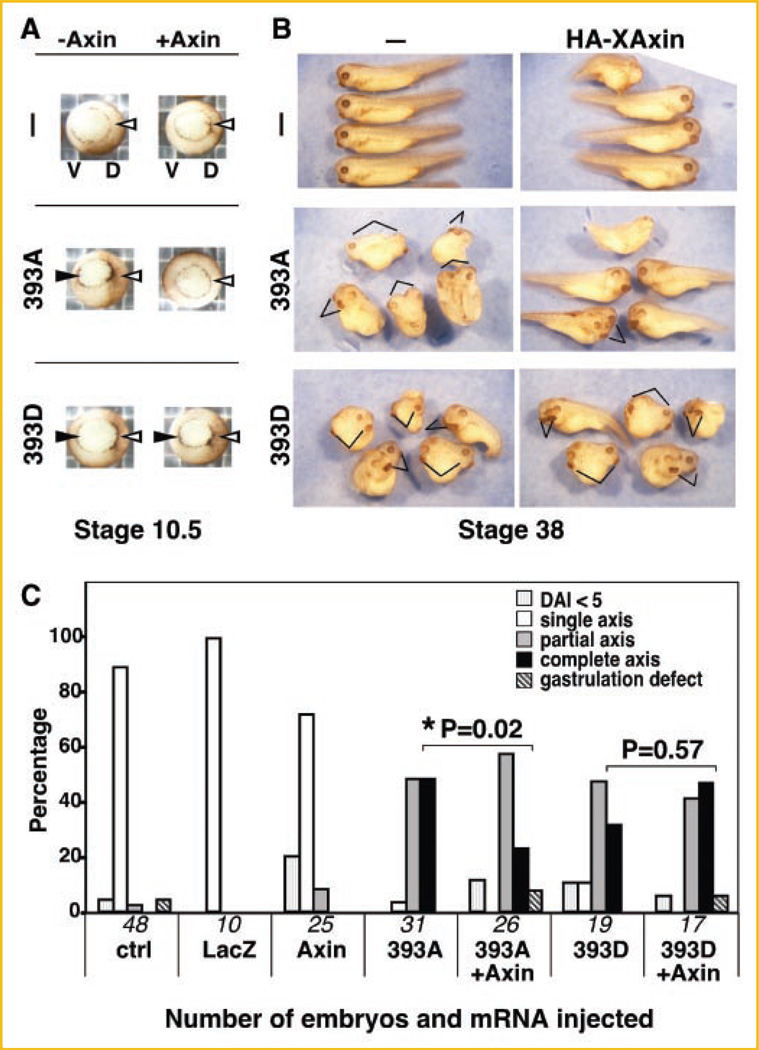
To demonstrate that 393D myc-β-catenin was not regulated by HA-XAxin in vivo, a bioassay in X. laevis that uses the induction of an ectopic dorsal axis as a measure of canonical Wnt signaling activation was utilized [Sokol, 1999]. For this, mRNAs for 393D or 393A myc-β-catenin were injected into stage 2–3 embryos with or without HA-XAxin mRNA. At stage 10.5, embryos display the characteristic of an endogenous dorsal axis: presence of the blastopore lip, an indentation in the dorsal side (D) of the embryo that marks the beginning of gastrulation (Fig. 5A, white arrowheads). Embryos that had received 393A or 393D mRNA exhibited an ectopic blastopore lip in the ventral side (V), characteristic of the induction of an ectopic site of gastrulation that will lead to an ectopic embryonic axis (Fig. 5A, black arrowheads). HA-XAxin inhibits ectopic blastopore lip formation induced by 393A but not 393D mRNA (Fig. 5A). At stage 38, control embryos have one single endogenous axis (Fig. 5B, top panels), embryos injected with 393A and 393D exhibited both endogenous and ectopic embryonic axis (lines, Fig. 5B). Correlating with the blastopore observations, HA-XAxin prevented the formation of an ectopic embryonic axis when co-injected with 393A but not 393D mRNA (lines, Fig. 5B). Quantitation of phenotypes at stage 38 (Fig. 5C), as described in Materials and Methods Section, showed that HA-XAxin inhibited ectopic axis induction by 393A myc-β-catenin (P = 0.02 by Fisher’s exact test) while 393D myc-β-catenin is resistant to Axin regulation (P = 0.57 by Fisher’s exact test). Thus, 393D β-catenin can elude downregulation by Axin in vivo.
Fig. 5.
Reduced regulation of T393 pseudophosphorylated β-catenin by Axin in vivo: Ectopic axis induction by 393D β-catenin is not affected by Axin. Two nanograms of mRNAs for 393A or 393D myc-β-catenin were ventrally injected in Xenopus laevis embryos with or without 1.2 ng of HA-XAxin coinjection. A: Vegetal view of stage 10.5 embryos showing endogenous (white arrowhead) and ectopic (black arrowhead) blastopore lips. The endogenous blastopore lip, dorsally located (D), is to the right; and ectopic blastopore lip, ventrally located (V), is to the left in all frames. B: Lateral view of embryos shown at stage 38. Note that both 393A and 393D induce an ectopic axis (indicated with lines). Top panels, HA-XAxin alone does not induce an ectopic axis. Middle panels, HA-XAxin prevents induction of an ectopic axis by 393A. Lower panels, HA-XAxin does not affect the formation of an ectopic axis induced by 393D. Some embryos injected with HA-XAxin were ventralized (top and middle panels on the right column), as observed by other researchers [Fukui et al., 2000] and in rescue experiments of other Wnt components [Zeng et al., 1997]. C: Histogram showing pooled data from four independent experiments. Embryos were categorized and scored as described in Materials and Methods. LacZ mRNA-injected and uninjected controls (ctrl) are included. Numbers on the X-axis reflect number of embryos in each treatment. Asterisk (*) indicates P < 0.05.
REDUCED PHOSPHORYLATION OF PHOSPHOMIMETIC MYC-β-CATENIN BY CKI/GSK3β
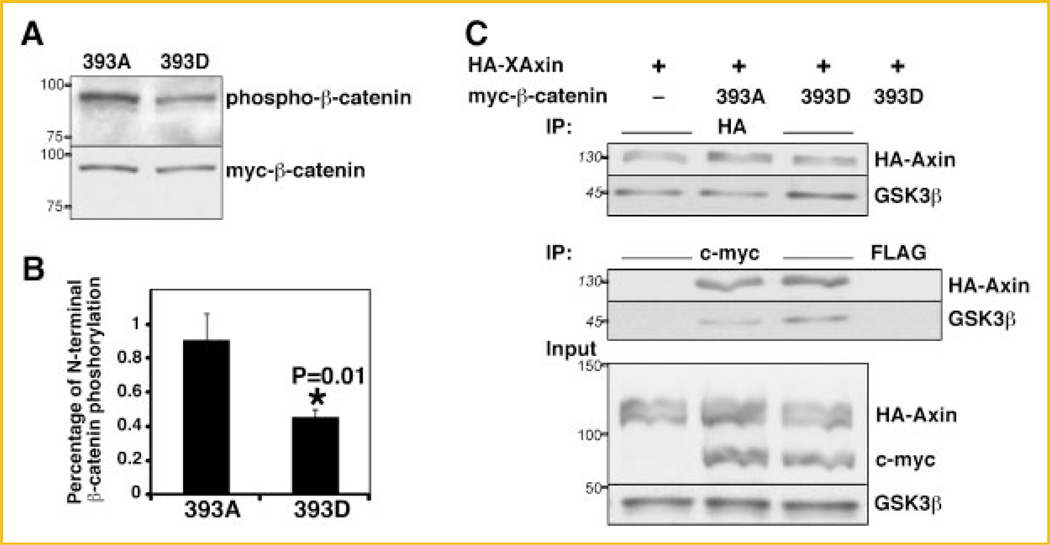
To determine whether the reduced binding of 393D β-catenin to Axin resulted in the expected decreased phosphorylation by GSK3β, we used a specific antibody that recognizes phosphorylation of β-catenin by GSK3β on Ser33, Ser37 and Thr41. To perform this experiment we expressed both β-catenin mutants in C57MG cells. When expressed at similar levels, 393D myc-β-catenin was phosphorylated by GSK3β to a lesser extent than 393A myc-β-catenin (Fig. 6A). Quantitation of three independent experiments showed that the ratio of GSK3β-phosphorylated to total 393D mycβ-catenin (0.45 ± 0.17) was about half that of 393A myc-β-catenin (0.9 ± 0.05) (Fig. 6B). The reduced GSK3β phosphorylation of 393D myc-β-catenin relative to that of 393A (P = 0.01, Fig. 6B) directly correlated with its reduced binding to Axin relative to 393A (Fig. 4D). These data indicate that β-catenin phosphorylated at T393 is less phosphorylated by CK1/GSK3β due to reduced affinity to Axin.
Fig. 6.
Reduced phosphorylation of 393D β-catenin by CKI/GSK3β. A: C57MG cells were lysed 24 h after transfection with 393D or 393A myc-β-catenin or pCS2 (not shown). Cell lysates were subjected to immunoblotting with the indicated antibodies. B: Histogram representing quantitation from three independent experiments showing the relative N-terminal phosphorylation of myc-β-catenin mutants relative to their expression. Asterisk (*) indicates P < 0.05. C: Higher affinity of endogenous GSK3β for Axin in HEK293T cells expressing 393D β-catenin. The experimental protocol was the same as in Figure 4C. Expression levels of exogenous and endogenous proteins are shown in the lower panel (input). Endogenous GSK3β levels are not affected by β-catenin transfection. Representative immunoblots from four independent experiments. Indicated on the left of the immunoblots are the apparent molecular weights of the proteins (numbers in italics) or the position and weight of the molecular markers utilized (numbers in roman).
ENHANCED GSK3β BINDING TO AXIN IN THE PRESENCE OF T393 PHOSPHOMIMETIC β-CATENIN
We tested whether GSK3β is present in similar amounts in the complexes between HA-XAxin and 393D myc-β-catenin or 393A myc-β-catenin. To perform this experiment we transfected HEK293T cells with HA-Axin and the β-catenin mutants in three independent experiments. Unexpectedly, endogenous GSK3β co-immunoprecipitated with HA-XAxin at higher levels in the presence of 393D myc-β-catenin than with 393A myc-β-catenin (Fig. 6C, upper panel). Similar results were found in the reverse IP (Fig. 6C, middle panel). Direct binding between 393D or 393A and GSK3β in the absence of expressed HA-Axin was not detectable (data not shown), as reported by other researchers [Ikeda et al., 1998]. This could be due to low endogenous levels of the scaffold protein Axin and/or low expression levels of myc-tagged mutants (Fig. 3). To test whether, in cells expressing 393D, the increase in GSKβ in the Axin complexes was due to a global increase in GSK3β expression, we analyzed total endogenous GSK3β levels. Endogenous GSK3β levels were not affected by expression of any of the exogenous β-catenin mutants (Fig. 6C, input), suggesting that this is a specific effect on the destruction complex upon expression of 393D β-catenin.
ENHANCED BINDING OF GSK3β TO AXIN IN THE PRESENCE OF ACTIVATED FORMS OF β-CATENIN
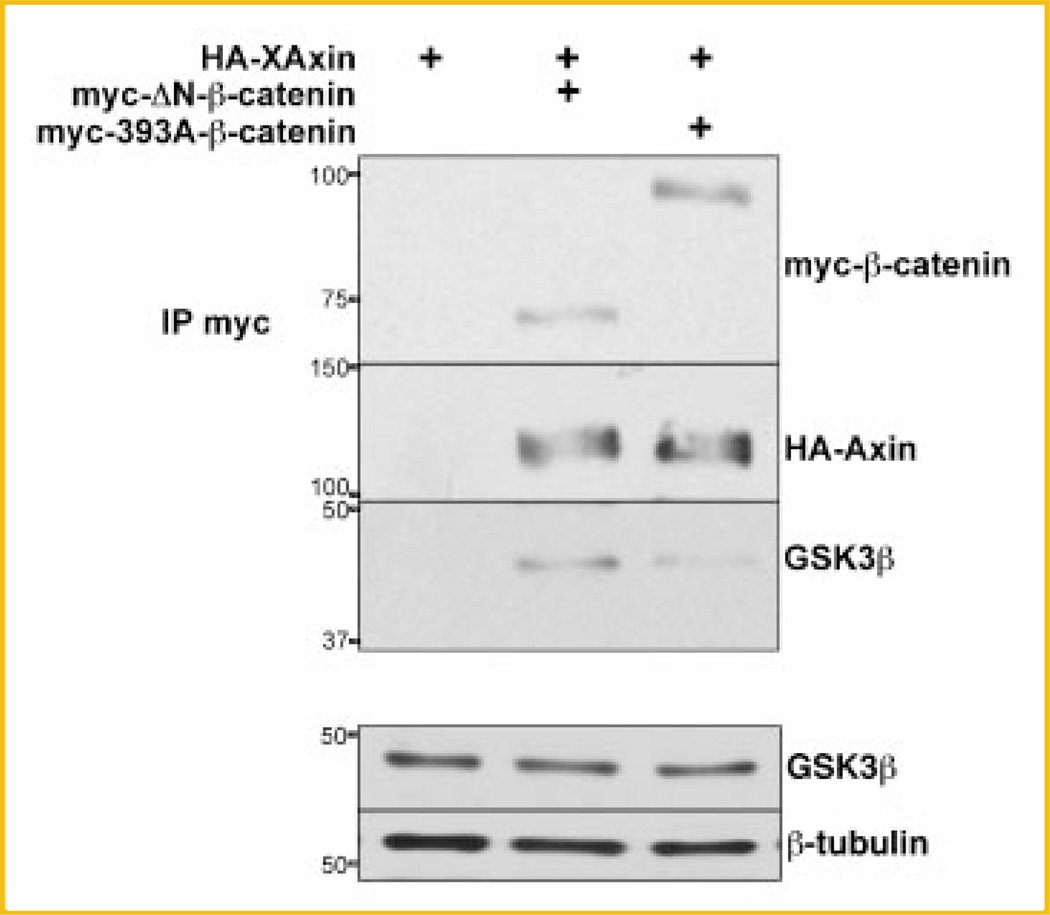
One possible explanation for the elevated level of GSK3β in HA-Axin immunoprecipitates in the presence of 393D myc-β-catenin is that the destruction complex may be more efficiently assembled in the presence of activated forms of β-catenin. To test this hypothesis, we compared the binding of endogenous GSK3β to myc-ΔN-β-catenin, a stabilized activated form of β-catenin that does not have the CK1/GSK3β phosphorylation or β-TrCP ubiquitination sites [Aberle et al., 1997; Amit et al., 2002; Yanagawa et al., 2002], to that of the 393A myc-β-catenin mutant. HEK293T cells were transfected with 393A myc-β-catenin or myc-ΔN-β-catenin, either alone or with HA-XAxin (Fig. 7). Extracts from these transfected cells were subjected to IP with anti-myc antibodies. Results from three independent experiments show that myc-ΔN-β-catenin behaved the same way as 393D myc-β-catenin. Specifically, HA-XAxin bound less myc-ΔN-β-catenin than 393A myc-β-catenin; moreover, HA-XAxin bound more endogenous GSK3β in the presence of myc-ΔN-β-catenin than in the presence of 393A myc-β-catenin (Fig. 7). Endogenous GSK3β levels were not affected by expression of myc-ΔN-β-catenin (Fig. 7, input). This similarity between ΔN-β-catenin and 393D (Figs. 6 and 7) together with its increased stability and enhanced transactivation potential indicates that β-catenin phosphorylated at T393 behaves like an active form of β-catenin.
Fig. 7.
Endogenous GSK3β co-immunoprecipitates more with Axin in HEK293T cells expressing a myc-tagged N-terminal deleted β-catenin. The experimental protocol was the same as in Figure 4C. Cell lysates were subjected to IP with anti-c-myc antibodies. GSK3β levels are not affected by β-catenin transfection (lower panel). Representative immunoblots from three independent experiments. Position and weight of the molecular markers utilized is indicated on the left of the immunoblots.
DISCUSSION
β-Catenin is a key protein involved in Wnt signaling and in adhesion that has an essential role in embryonic development [Haegel et al., 1995; Huelsken et al., 2000] and is found dysregulated in human cancers [Waltzer and Bienz, 1999]. CK2, a serine-threonine kinase necessary for embryonic development and implicated in cancer, interacts with β-catenin although their relationship remains elusive [Seldin et al., 2005]. In vitro, CK2 phosphorylates β-catenin at its N-terminus [Bek and Kemler, 2002] and in its armadillo region [Song et al., 2003]. While CK2 phosphorylation of β-catenin at it N-terminus is proposed to destabilize β-catenin, phosphorylation of β-catenin in its armadillo region is proposed to stabilize it. Since stabilization of β-catenin is a key event in Wnt signaling, this work has focused on determining whether phosphorylation in the armadillo region, at T393, is sufficient to stabilize β-catenin. In this report, we have shown that this is the case, as pseudophosphorylation of β-catenin at T393 is sufficient to stabilize β-catenin. Phosphorylation of T393 is of functional significance as 393D β-catenin expression has enhanced transactivation potential. This increase in Wnt-target gene expression seems to be direct, and not through enhanced stabilization of endogenous β-catenin. Our data show that phosphorylation of β-catenin at T393 increased stability of β-catenin through decreased affinity to Axin and not through sequestration and protection in the more stable cadherin pool [Papkoff, 1997]. This stabilization mechanism is consistent with a key role for T393 in the regulation of Wnt signaling.
Taken together, the published data suggests that CK2 plays two distinct roles in β-catenin function, one on cell adhesion and one for canonical Wnt signaling [Daugherty and Gottardi, 2007]. CK2 may regulate cell adhesion by phosphorylating two of its components, cadherin and β-catenin. In this regard, CK2 may play a critical role in the maintenance of epidermal cohesion as CK2 co-immunoprecipitates and co-localizes with E-cadherin [Serres et al., 2000], CK2 phosphorylates E-cadherin in vitro [Serres et al., 2000] and phosphorylation of E-cadherin by CK2 leads to increased binding affinity for β-catenin [Lickert et al., 2000; Catimel et al., 2006]. Several CK2 putative phosphorylation sites in the cytoplasmic portion of E-cadherin have been identified [Lickert et al., 2000; Huber and Weis, 2001]. However, the function of these sites and their dependence on CK2 phosphorylation in vivo has not yet been described. CK2 also phosphorylates β-catenin at S29/T102/T112 in vitro [Bek and Kemler, 2002]. Phosphorylation of these residues may regulate the localization of the heterodimer β-catenin/α-catenin as immunofluorescence studies show that an A29/A102/A112 mutant of β-catenin redistributed to the cytoplasm in correlation with α-catenin redistribution [Bek and Kemler, 2002]. The A29/A102/A112 β-catenin mutant is largely localized in the cytoplasm and thus, it could be a target of the destruction complex, however A29/A102/A112 β-catenin has enhanced stability correlating with decreased affinity to Axin [Bek and Kemler, 2002]. One possible explanation for this decreased Axin binding could be that β-catenin is not accessible to Axin when bound to α-catenin in a heterodimer. The dependence of S29/T102/T112 for CK2 phosphorylation in vivo has not yet been studied.
In addition, CK2 may play an inducible positive role in Wnt signaling, as CK2 activity is upregulated by Wnt in mammalian cell lines in culture [Gao and Wang, 2006]. CK2 substrates and interactors include β-catenin, dishevelled, APC and TCF [Willert et al., 1997; Song et al., 2000; Zhang et al., 2001; Miravet et al., 2002]. β-catenin maybe a major target of CK2 action on canonical Wnt signaling [Song et al., 2000; Dominguez et al., 2004], as CK2 plays a role in stabilizing β-catenin through phosphorylation of T393 ([Song et al., 2000, 2003; Dominguez et al., 2004] and this work). In addition, CK2 has been shown to regulate the interaction and transactivation activity of a β-catenin/LEF-1 complex through phosphorylation of LEF-1 at S42, S61 [Hammerlein et al., 2005; Wang and Jones, 2006]. The interaction between CK2 and APC seems to regulate cell cycle progression [Homma et al., 2002; Hildesheim et al., 2005; Homma and Homma, 2005], but it has not been determined if it also affects Wnt signaling. On the other hand, phosphorylation of dishevelled by CK2 seems to play a key role in the regulation of non-canonical Wnt signaling pathways [Bryja et al., 2008].
Our results also suggest that T393 phosphorylation does not act as a switch between the adhesion role of β-catenin and the signaling function [Heasman et al., 1994; Orsulic et al., 1999; Gottardi and Gumbiner, 2004; Bernard et al., 2008]. It is possible that phosphorylation of T393 in β-catenin may introduce a structural change in this hinge region of β-catenin [Huber et al., 1997], leading to decreased affinity to Axin without change in the binding to E-cadherin. Indeed, structural studies show that E-cadherin and XTCF3, but not Axin, share essentially the same sets of binding residues in β-catenin [Huber and Weis, 2001]. Alternatively, since T393 is located in the armadillo repeat region of β-catenin that binds Axin [Huber and Weis, 2001; Choi et al., 2006], the phosphorylation of this site may prevent direct binding without conformational change.
Intriguingly, in the presence of T393 phosphorylated β-catenin, there is an increase of GSK3β levels in the destruction complex. The same phenomenon was observed with an oncogenic N-terminal deleted form of β-catenin. Since we did not observe a change in the total level of GSK3β in either case, it is plausible that the increased GSK3β level in the destruction complex in cells expressing active forms of β-catenin reflects a feedback mechanism designed to suppress the more stable forms of β-catenin, such as 393D or ΔN-β-catenin. The mechanism for this increased binding of GSK3β to Axin is unknown and it may be different from the published regulation of the GSK3β-Axin complex by Wnt though phosphorylation/dephosphorylation and subcellular relocalization [Strovel et al., 2000; Lee et al., 2003; Tolwinski et al., 2003; Liu et al., 2005; Luo et al., 2007].
In our experiments, analysis of the effects of the wildtype protein proved difficult, as there was high variability between experiments. Our data (Fig. 1) suggest that wildtype β-catenin exists in both phosphorylated and un-phosphorylated states in vivo. The ratio of T393 phosphorylated to unphosphorylated β-catenin may depend upon the phase of the cell cycle, as β-catenin is regulated by growth factors present in serum [Chen et al., 2000; Olmeda et al., 2003]. Similarly, CK2 activity is also regulated during the cell cycle [Bosc et al., 1999; Homma and Homma, 2008], so it is conceivable that the regulation of β-catenin by serum may be dependent upon CK2 phosphorylation. Thus, in this work we limited our scope to determine how pseudophosphorylation of T393 affects β-catenin function compared to unphosphorylated β-catenin. Future experiments will determine if there is direct effect of CK2 on β-catenin during the cell cycle and whether this effect is T393 dependent.
Taken together, our data are consistent with a model in which in the absence of Wnt signaling, constitutively active CK1 and GSK2β contribute to cytoplasmic β-catenin degradation. In the presence of Wnt, CK2 is activated leading to phosphorylation of β-catenin at T393. Phosphorylation of β-catenin at T393 rescues β-catenin from the destruction complex, effectively stabilizing the protein and contributing to its activation. Thus, it is our expectation that upon Wnt signaling, β-catenin will be phosphorylated at T393, and that this phosphorylation is dependent on CK2 activity. Of note, direct identification of T393 phosphorylation in vivo by metabolic labeling and by mass spectrometry analysis have not been successful thus far (data not shown). The size of the protein, the highly compacted structure of the armadillo repeats which are resistant to protease digestion, and the potential complex phosphorylation pattern has made this effort difficult. That could explain why, to our knowledge, only two in vivo phosphorylation sites in β-catenin have been directly identified by those techniques, S45 by CK1 [Amit et al., 2002], and S552 by Akt [Fang et al., 2007]. Determining if T393 is phosphorylated in vivo upon Wnt stimulation, and its dependence on CK2 phosphorylation in vivo are subjects of ongoing study in our laboratories.
ACKNOWLEDGMENTS
We are grateful to B. Gumbiner, W. Birchmeier, R. Moon, X. He, and R.A. Shivdasani for providing plasmids. We thank M. Malikova and E. Smith for help obtaining embryos and C. Bresilla, J. Cha and P. Hogan for frog husbandry. We thank G. Sonenshein, S. Thiagalingam, J. Ward, B. Hovey, K. Chea and M. Hlavacova for critically reading the manuscript; the Developmental Interest Group and the Epithelial Group at BUSM for helpful discussions; Y. Zhang for help with statistical analysis and T. Khan for help with figures.
Grant sponsor: National Cancer Institute; Grant number: R01 CA71796; Grant sponsor: National Cancer Institute; Grant number: RO1 CA 87375; Grant sponsor: NIEHS; Grant number: P01 ES011624; Grant sponsor: AHA; Grant number: SDG 0735521T; Grant sponsor: Karin Grunebaum Cancer Research Foundation (Junior Faculty Award).
REFERENCES
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad KA, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2—A key suppressor of apoptosis. Adv Enzyme Regul. 2008;48:179–187. doi: 10.1016/j.advenzreg.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, Meissner RA. Casein kinase 2, circadian clocks, and the flight from mutagenic light. Mol Cell Biochem. 2005;274:141–149. doi: 10.1007/s11010-005-2943-1. [DOI] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: A molecular switch for the Wnt pathway. Genes Dev. 2002;16:1066–1076. doi: 10.1101/gad.230302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bek S, Kemler R. Protein kinase CKII regulates the interaction of beta-catenin with alpha-catenin and its protein stability. J Cell Sci. 2002;115:4743–4753. doi: 10.1242/jcs.00154. [DOI] [PubMed] [Google Scholar]
- Bernard P, Fleming A, Lacombe A, Harley VR, Vilain E. Wnt4 inhibits beta-catenin/TCF signaling by redirecting beta-catenin to the cell membrane. Biol Cell. 2008;100:167–177. doi: 10.1042/BC20070072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosc DG, Luscher B, Litchfield DW. Expression and regulation of protein kinase CK2 during the cell cycle. Mol Cell Biochem. 1999;191:213–222. [PubMed] [Google Scholar]
- Bryja VE, Schambony A, Cajanek LS, Dominguez I, Arenas E, Schulte G. Beta-Arrestin and casein kinase 1/2 define distinct branches of non-canonical WNT signalling pathways. EMBO Rep. 2008;9:1244–1250. doi: 10.1038/embor.2008.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchou T, Vernet M, Blond O, Jensen HH, Pointu H, Olsen BB, Cochet C, Issinger OG, Boldyreff B. Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol. 2003;23:908–915. doi: 10.1128/MCB.23.3.908-915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton DA, Litchfield DW. The shape of things to come: An emerging role for protein kinase CK2 in the regulation of cell morphology and the cytoskeleton. Cell Signal. 2006;18:267–275. doi: 10.1016/j.cellsig.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Catimel B, Layton M, Church N, Ross J, Condron M, Faux M, Simpson RJ, Burgess AW, Nice EC. In situ phosphorylation of immobilized receptors on biosensor surfaces: Application to E-cadherin/beta-catenin interactions. Anal Biochem. 2006;357:277–288. doi: 10.1016/j.ab.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Chen RH, Ding WV, McCormick F. Wnt signaling to beta-catenin involves two interactive components. Glycogen synthase kinase-3beta inhibition and activation of protein kinase C. J Biol Chem. 2000;275:17894–17899. doi: 10.1074/jbc.M905336199. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Huber AH, Weis WI. Thermodynamics of beta-catenin-ligand interactions: The roles of the N- and C-terminal tails in modulating binding affinity. J Biol Chem. 2006;281:1027–1038. doi: 10.1074/jbc.M511338200. [DOI] [PubMed] [Google Scholar]
- Cox RT, Pai LM, Miller JR, Orsulic S, Stein J, McCormick CA, Audeh Y, Wang W, Moon RT, Peifer M. Membrane-tethered Drosophila Armadillo cannot transduce Wingless signal on its own. Development. 1999;126:1327–1335. doi: 10.1242/dev.126.6.1327. [DOI] [PubMed] [Google Scholar]
- Currier N, Solomon SE, Demicco EG, Chang DL, Farago M, Ying H, Dominguez I, Sonenshein GE, Cardiff RD, Xiao ZX, Sherr DH, Seldin DC. Oncogenic signaling pathways activated in DMBA-induced mouse mammary tumors. Toxicol Pathol. 2005;33:726–737. doi: 10.1080/01926230500352226. [DOI] [PubMed] [Google Scholar]
- Daugherty RL, Gottardi CJ. Phospho-regulation of Beta-catenin adhesion and signaling functions. Physiology (Bethesda) 2007;22:303–309. doi: 10.1152/physiol.00020.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez I, Mizuno J, Wu H, Song DH, Symes K, Seldin DC. Protein kinase CK2 is required for dorsal axis formation in Xenopus embryos. Dev Biol. 2004;274:110–124. doi: 10.1016/j.ydbio.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Dominguez I, Mizuno J, Wu H, Imbrie GA, Symes K, Seldin DC. A role for CK2alpha/beta in Xenopus early embryonic development. Mol Cell Biochem. 2005;274:125–131. doi: 10.1007/s11010-005-3073-5. [DOI] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui A, Kishida S, Kikuchi A, Asashima M. Effects of rat Axin domains on axis formation in Xenopus embryos. Dev Growth Differ. 2000;42:489–498. doi: 10.1046/j.1440-169x.2000.00536.x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Wang HY. Casein kinase 2 Is activated and essential for Wnt/beta-catenin signaling. J Biol Chem. 2006;281:18394–18400. doi: 10.1074/jbc.M601112200. [DOI] [PubMed] [Google Scholar]
- Giarre M, Semenov MV, Brown AM. Wnt signaling stabilizes the dual-function protein beta-catenin in diverse cell types. Ann NY Acad Sci. 1998;857:43–55. doi: 10.1111/j.1749-6632.1998.tb10106.x. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 2004;167:339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol. 2001;153:1049–1060. doi: 10.1083/jcb.153.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra B, Issinger OG. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis. 1999;20:391–408. doi: 10.1002/(SICI)1522-2683(19990201)20:2<391::AID-ELPS391>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyenis L, Litchfield DW. The emerging CK2 interactome: Insights into the regulation and functions of CK2. Mol Cell Biochem. 2008;316:5–14. doi: 10.1007/s11010-008-9830-5. [DOI] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- Hagen T, Sethi JK, Foxwell N, Vidal-Puig A. Signalling activity of beta-catenin targeted to different subcellular compartments. Biochem J. 2004;379:471–477. doi: 10.1042/BJ20031749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerlein A, Weiske J, Huber O. A second protein kinase CK1-mediated step negatively regulates Wnt signalling by disrupting the lymphocyte enhancer factor-1/beta-catenin complex. Cell Mol Life Sci. 2005;62:606–618. doi: 10.1007/s00018-005-4507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Hildesheim J, Salvador JM, Hollander MC, Fornace AJ., Jr Casein kinase 2- and protein kinase A-regulated adenomatous polyposis coli and beta-catenin cellular localization is dependent on p38 MAPK. J Biol Chem. 2005;280:17221–17226. doi: 10.1074/jbc.M410440200. [DOI] [PubMed] [Google Scholar]
- Homma MK, Homma Y. Regulatory role of CK2 during the progression of cell cycle. Mol Cell Biochem. 2005;274:47–52. doi: 10.1007/s11010-005-3111-3. [DOI] [PubMed] [Google Scholar]
- Homma MK, Homma Y. Cell cycle and activation of CK2. Mol Cell Biochem. 2008;316:49–55. doi: 10.1007/s11010-008-9823-4. [DOI] [PubMed] [Google Scholar]
- Homma MK, Li D, Krebs EG, Yuasa Y, Homma Y. Association and regulation of casein kinase 2 activity by adenomatous polyposis coli protein. Proc Natl Acad Sci USA. 2002;99:5959–5964. doi: 10.1073/pnas.092143199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, He X. Wnt/beta-catenin signaling: New (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- Huber AH, Nelson WJ, Weis WI. Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell. 1997;90:871–882. doi: 10.1016/s0092-8674(00)80352-9. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius MA, Rai SD, Kitajewski J. Chimeric Wnt proteins define the amino-terminus of Wnt-1 as a transformation-specific determinant. Oncogene. 1999;18:149–156. doi: 10.1038/sj.onc.1202268. [DOI] [PubMed] [Google Scholar]
- Kao KR, Elinson RP. The entire mesodermal mantle behaves as Spemann’s organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001;20:3247–3257. doi: 10.1038/sj.onc.1204411. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickert H, Bauer A, Kemler R, Stappert J. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/beta-catenin interaction and strengthens cell-cell adhesion. J Biol Chem. 2000;275:5090–5095. doi: 10.1074/jbc.275.7.5090. [DOI] [PubMed] [Google Scholar]
- Litchfield DW. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Lou DY, Dominguez I, Toselli P, Landesman-Bollag E, O’Brien C, Seldin DC. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol Cell Biol. 2008;28:131–139. doi: 10.1128/MCB.01119-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R, Shabanowitz J, Hunt DF, Yost HJ, Virshup DM. Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. EMBO J. 2007;26:1511–1521. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- Miller JR, Moon RT. Analysis of the signaling activities of localization mutants of beta- catenin during axis specification in Xenopus. J Cell Biol. 1997;139:229–243. doi: 10.1083/jcb.139.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miravet S, Piedra J, Miro F, Itarte E, Garcia de Herreros A, Dunach M. The transcriptional factor Tcf-4 contains different binding sites for beta-catenin and plakoglobin. J Biol Chem. 2002;277:1884–1891. doi: 10.1074/jbc.M110248200. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: Diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos. I. characterization and timing of cellular changes at the midblastula stage. Cell. 1982;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis. Amsterdam: North-Holland Publishing Company; 1967. [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Olmeda D, Castel S, Vilaro S, Cano A. Beta-catenin regulation during the cell cycle: Implications in G2/M and apoptosis. Mol Biol Cell. 2003;14:2844–2860. doi: 10.1091/mbc.E03-01-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112:1237–1245. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- Papkoff J. Regulation of complexed and free catenin pools by distinct mechanisms. Differential effects of Wnt-1 and v-Src. J Biol Chem. 1997;272:4536–4543. [PubMed] [Google Scholar]
- Provost E, Rimm DL. Controversies at the cytoplasmic face of the cadherin-based adhesion complex. Curr Opin Cell Biol. 1999;11:567–572. doi: 10.1016/s0955-0674(99)00015-0. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Seldin DC, Landesman-Bollag E, Farago M, Currier N, Lou D, Dominguez I. CK2 as a positive regulator of Wnt signalling and tumourigenesis. Mol Cell Biochem. 2005;274:63–67. doi: 10.1007/s11010-005-3078-0. [DOI] [PubMed] [Google Scholar]
- Serres M, Filhol O, Lickert H, Grangeasse C, Chambaz EM, Stappert J, Vincent C, Schmitt D. The disruption of adherens junctions is associated with a decrease of E-cadherin phosphorylation by protein kinase CK2. Exp Cell Res. 2000;257:255–264. doi: 10.1006/excr.2000.4895. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signalling. Genes Cells. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Wnt signaling and dorso-ventral axis specification in vertebrates. Curr Opin Genet Dev. 1999;9:405–410. doi: 10.1016/S0959-437X(99)80061-6. [DOI] [PubMed] [Google Scholar]
- Somorjai IM, Martinez-Arias A. Wingless signalling alters the levels, subcellular distribution and dynamics of Armadillo and E-cadherin in third instar larval wing imaginal discs. PLoS ONE. 2008;3:e2893. doi: 10.1371/journal.pone.0002893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DH, Sussman DJ, Seldin DC. Endogenous protein kinase CK2 participates in Wnt Signaling in mammary epithelial cells. J Biol Chem. 2000;275:23790–23797. doi: 10.1074/jbc.M909107199. [DOI] [PubMed] [Google Scholar]
- Song DH, Dominguez I, Mizuno J, Kaut M, Mohr SC, Seldin DC. CK2 phosphorylation of the armadillo repeat region of beta-catenin potentiates Wnt signaling. J Biol Chem. 2003;278:24018–24025. doi: 10.1074/jbc.M212260200. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Burgering BM, van de Wetering M, Clevers HC. Tcf-1-mediated transcription in T lymphocytes: Differential role for glycogen synthase kinase-3 in fibroblasts and T cells. Int Immunol. 1999;11:317–323. doi: 10.1093/intimm/11.3.317. [DOI] [PubMed] [Google Scholar]
- Strovel ET, Wu D, Sussman DJ. Protein phosphatase 2Calpha dephosphorylates axin and activates LEF-1-dependent transcription. J Biol Chem. 2000;275:2399–2403. doi: 10.1074/jbc.275.4.2399. [DOI] [PubMed] [Google Scholar]
- Tapia JC, Torres VA, Rodriguez DA, Leyton L, Quest AF. Casein kinase 2 (CK2) increases survivin expression via enhanced beta-catenin-T cell factor/lymphoid enhancer binding factor-dependent transcription. Proc Natl Acad Sci USA. 2006;103:15079–15084. doi: 10.1073/pnas.0606845103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4:407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Vaidya AB, Lasfargues EY, Sheffield JB, Coutinho WG. Murine mammary tumor virus (MuMTV) infection of an epithelial cell line established from C57BL/6 mouse mammary glands. Virology. 1978;90:12–22. doi: 10.1016/0042-6822(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Waltzer L, Bienz M. The control of beta-catenin and TCF during embryonic development and cancer. Cancer Metastasis Rev. 1999;18:231–246. doi: 10.1023/a:1006321324190. [DOI] [PubMed] [Google Scholar]
- Wang S, Jones KA. CK2 controls the recruitment of Wnt regulators to target genes in vivo. Curr Biol. 2006;16:2239–2244. doi: 10.1016/j.cub.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Willert K, Brink M, Wodarz A, Varmus H, Nusse R. Casein kinase 2 associates with and phosphorylates dishevelled. EMBO J. 1997;16:3089–3096. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Toselli PA, Russell LD, Seldin DC. Globozoospermia in mice lacking the casein kinase II alpha’ catalytic subunit. Nat Genet. 1999;23:118–121. doi: 10.1038/12729. [DOI] [PubMed] [Google Scholar]
- Yanagawa S, Matsuda Y, Lee JS, Matsubayashi H, Sese S, Kadowaki T, Ishimoto A. Casein kinase I phosphorylates the Armadillo protein and induces its degradation in Drosophila. EMBO J. 2002;21:1733–1742. doi: 10.1093/emboj/21.7.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zhang F, White RL, Neufeld KL. Cell density and phosphorylation control the subcellular localization of adenomatous polyposis coli protein. Mol Cell Biol. 2001;21:8143–8156. doi: 10.1128/MCB.21.23.8143-8156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]