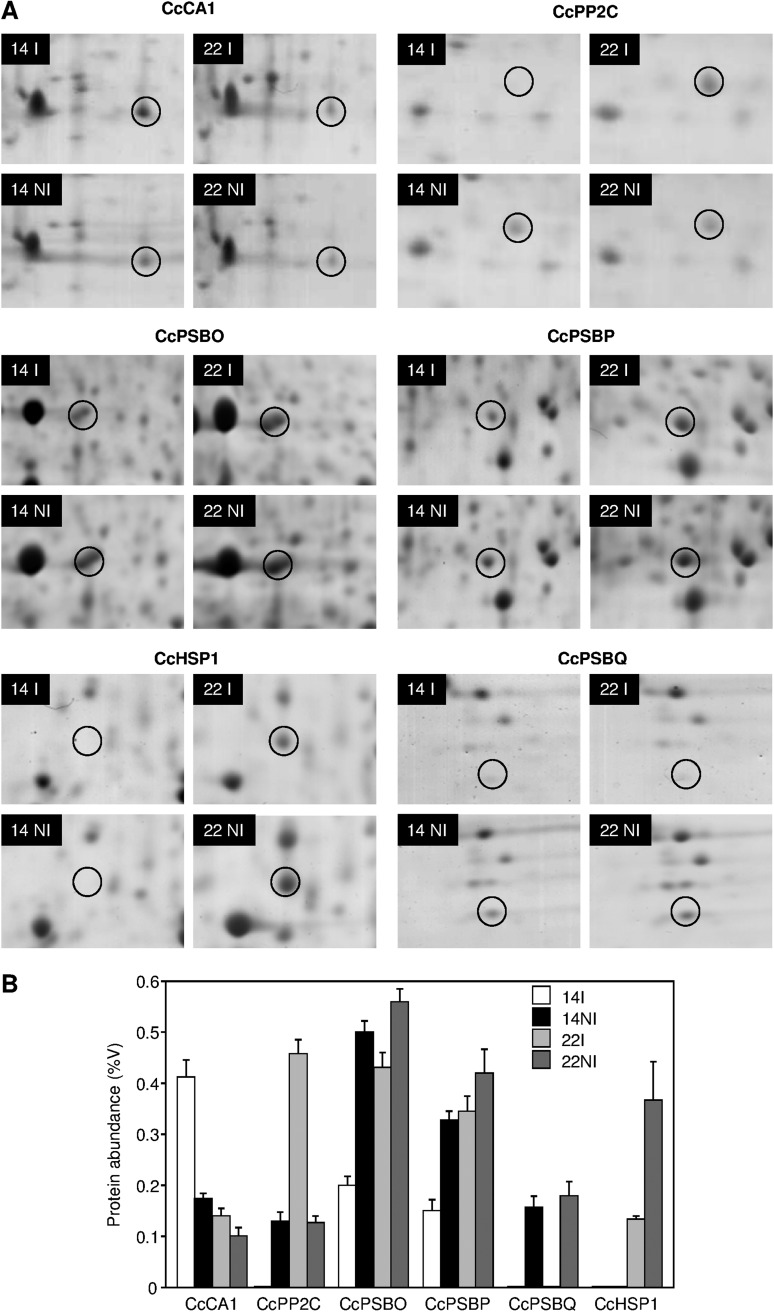
Fig. 3.
Protein responses in coffee leaf exposed to drought. (A) Plants were irrigated (I) or not irrigated (NI); leaf soluble proteins were extracted and separated by two-dimensional gel electrophoresis (2-DE), stained by Coomassie Brillant Blue, and scanned. The results shown are representative of at least three repetitions. Spots corresponding to CcCA1 (carbonic anhydrase) and CcPP2C (type-2C protein phosphatase) proteins were analysed using strips with an immobilized linear pH gradient of 3–10. Spots corresponding to CcPSBO (PSII oxygen evolving complex protein, OEC), CcPSBP (OEC), and CcHSP1 (small heat shock protein) proteins were analysed using strips with an immobilized linear pH gradient of 4–7, and CcPSBQ (OEC) with pH gradient 6–11. Proteins were further characterized by MALDI-TOF-MS/MS (see Table 2). (B) Normalized protein abundance of the coffee leaves extracted from clones 14 and 22 of C. canephora subjected (NI) or not subjected (I) to drought. Protein abundance was deduced from the 2-DE using ImageMaster Platinum 6.0 software and is expressed as a percentage of volume (%V) which was calculated from the gel images as the volume of a specific spot divided by the sum of the volume of all other spots present in the gel multiplied by 100. Volume percentage averages were calculated from 2-DE gels loaded with three biological replicates.