Figure 1. Structural aspects of the NPC and scaffold models.
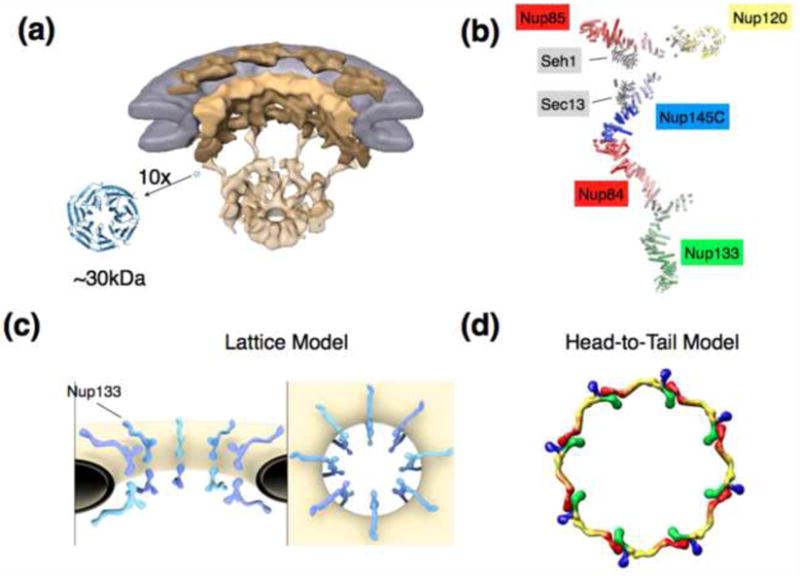
A) Overall architecture of the NPC determined by cryo-electron tomography and shown in a cut-away view. The nuclear envelope is colored in grey, scaffolding components of the NPC depicted in shades of yellow [16]. For scale, a canonical β-propeller in 10fold magnification is shown. β-Propellers, together with various helical units, make up a large portion of the stable NPC scaffold. Fitting of crystal structures into the cryo-ET structures is unreliable due to the resolution gap.
B) Composite structure of the heptameric 575 kDa Y-complex, a major scaffolding component of the NPC. The composite is generated based on individual crystal structures [18-22], modeling [31], interaction data, and EM pictures [17,39].
C) Arrangement of the Y-complexes within the NPC as suggested by the lattice model [18,20]. Two Y-complexes are related by a 2-fold symmetry axis and span the width of the NPC, with Nup133 pointing outward. Likely 8 such pairs, consistent with the 8fold rotational symmetry of the NPC, are positioned around the central transport axis of the NPC. Whether or not the two Y complexes directly bind or not is still unresolved. Lateral connection between Y-complexes most likely occurs via other components of the NPC, but this is also not determined yet.
D) Alternative model for the Y-complex arrangement within the pore. 8 Y-complexes are directly connected in head-to-tail fashion and form a ring [5,24,44,46]. Some authors argue for an arrangement with 2 stacked rings [5], others for a configuration with 4 stacked rings per NPC [24,44]. Color coding: Nup133 (red), Nup84/Nup145C/Sec13 (yellow), Nup85/Seh1 (blue), Nup120 (green).
