1. Background
Adolescent depression is a serious public health concern. It is estimated that 15% to 30% of adolescents will experience an episode of major depressive disorder (Seeley & Lewinsohn, 2008). Major depressive disorder is a leading risk factor for suicide (Tomson, Pangrazi, Friedman, & Hutchison, 2003), has been implicated as a contributor to substance abuse and delinquency, and has been found to negatively affect interpersonal relationships and academic performance (Jerstad, Boutelle, Ness, & Stice, 2010; Klein, Torpey, Bufferd, & Dyson, 2008). The prevalence of major depressive disorder increases during early to mid-adolescence, with the most dramatic increases evident for girls (Garber, Keiley, & Martin, 2002; Hankin, 2006).
Another potent public health concern occurring during the same developmental period is the significant decline in physical activity as children age (Lotan, Merrick, & Carmeli, 2004; Whitt-Glover et al., 2009). There is a particularly sharp decline through the teenage years (Kimm et al., 2000), especially for girls (Whitt-Glover et al., 2009), with ages 11-12 believed to be a critical point at which physical activity begins to diminish (Brodersen, Steptoe, Williamson, & Wardle, 2005).
Studies of youth and adolescents suggest a negative relationship between depressive symptoms and physical activity (e.g., Boone & Leadbeater, 2006; Crews, Lochbaum, & Landers, 2004; Donaldson & Ronan, 2006); however, research during this developmental period is limited. A review by Larun, Nordheim, Ekeland, Hagen, and Heian (2009) concluded that the relationships between physical activity and youth depressive symptoms are generally unknown because the evidence base is extremely scarce. Most research in this area has used cross-sectional designs, and focused on college-age students or adults. Few studies have assessed the relationships between adolescent depressive symptoms and physical activity, and even fewer have used longitudinal data (Birkeland, Torsheim, & Wold, 2009; Jerstad et al., 2010; Motl, Birnbaum, Yubik, & Dishman, 2004). Findings from the limited longitudinal research have been mixed. For example, Jerstad et al. (2010) found that early physical activity reduced the risk for future increases in depressive symptoms as well as the onset of major or minor depressive disorder in adolescence, whereas Birkeland, Torsheim, and Wold (2009) concluded that adolescent leisure-time physical activity and depressive symptoms covary, but they found no prospective relations between physical activity and depressive symptoms.
A number of factors appear to influence adolescent physical activity and depressive symptoms, but more etiological and developmental longitudinal research is needed to understand the nature of these influences. Potentially influential variables related to physical activity and depressive symptoms examined in the present research are family, physiological, and demographic factors.
1.1. Family factors
Family factors have been shown to separately influence both adolescent physical activity and depressive symptoms or disorders. Parents, for example, influence their children's physical activity directly and indirectly, with mechanisms hypothesized to account for consistencies in family activity inclusive of both genetics and environmental variables (e.g., modeling, shared activities, social support) (Duncan, Duncan, & Strycker, 2005; Welk, Wood, & Morss, 2003). Parental support appears to be particularly influential for adolescent physical activity (Beets, Vogel, Forlaw, Pitetti, & Cardinal, 2006; Roesch et al., 2009). Active parents tend to have more active children (Sallis, Taylor, Dowda, Freedson, & Pate, 2002), and physically active parents are likely to be more involved in their child's physical activities (Welk et al., 2003). Family factors related to adolescent depressive symptoms or disorders include low parental support, family conflict, parental depression, high levels of parental expressed emotion, and poor overall family functioning (Cui, Conger, & Lorenz, 2005; Lau, Rijsdijk, Gregory, McGuffin, & Eley, 2007; McCleary & Sanford, 2002; Seeley, Stice, & Rohde, 2009; Sheeber, Davis, Leve, Hops, & Tildesley, 2007).
1.2. Physiological factors
Puberty is a major physiologic event during adolescence that likely influences both physical activity (Baker, Birch, Trost, & Davison, 2007; Bradley, McMurray, Harrell, & Deng, 2000; Davison, Werder, Trost, Baker, & Birch, 2007) and vulnerability to depressive symptoms or disorders (Graber, Lewinsohn, Seeley, & Brooks-Gunn, 1997; Negriff & Susman, 2011). Physiological and developmental factors believed to be related to youth physical activity include body mass (e.g., body mass index [BMI]) and physical maturation (Berkey, Rockett, & Field, 2000; Brodersen et al., 2005). Few studies have explored the relations between BMI or physical maturation and adolescent physical activity, and physiological effects are unclear (Bradley et al., 2000). The limited research indicates possible differences for boys and girls, where advanced maturation is associated with less physical activity among girls and greater physical activity among boys (Baker, Birch, Trost, & Davison, 2007; Cumming et al., 2011). Physiological factors related to adolescent depressive symptoms or disorders include hormonal factors (Maxwell & Cole, 2009; Paikoff, Brooks-Gunn, & Warren, 1991), BMI (Cortese et al., 2009), weight change (Maxwell & Cole, 2009), body-image concerns (Stice, Hayward, Cameron, Killen, & Taylor, 2000), sleep disturbance (Dahl & Harvey, 2007), and eating disturbances (Maxwell & Cole, 2009; Stice et al., 2000; Seeley et al., 2009).
1.3. Demographic variables
Demographic variables are known to influence adolescent physical activity and depressive symptoms. Physical activity declines significantly during adolescence, with adolescent girls at particularly high risk for inactivity (Suris & Parera, 2005; Whitt-Glover et al., 2009). At the same time, there is an increase in depressive symptoms and disorders during adolescence, with girls particularly at risk (Hankin, 2006; Hammen, 2009; Seeley & Lewinsohn, 2008). More physical activity is reported in white compared to minority populations, and among children from higher-income families compared to lower-income families (Brodersen et al., 2005; Santos, Esculcas, & Mota, 2004; Whitt-Glover et al., 2009). Findings concerning various demographic associations with depressive symptoms or disorders have frequently been equivocal, including associations with race or ethnicity (Lewinsohn et al., 1997; Petersen et al., 1993; Rushton, Forcier, & Schectman, 2002; Seeley et al., 2009; Waschbusch, Sellers, LeBlanc, & Kelley, 2003), single versus dual parent household (Fergusson, Boden, & Horwood, 2007; Lewinsohn et al., 1997; Patten et al., 1997), parental education level (Goodman, Slap, & Huang, 2003; Kaltiala-Heino, Rimpelä, Rantanen, & Laippala, 2001; Lewinsohn et al., 1997; Rohde, Beevers, Stice, & O'Neil, 2009), and parental income or socioeconomic status (Goodman et al., 2003; Waschbusch et al., 2003). Methodological variations across studies, such as characteristics of the sampling area (Wight, Aneshensel, Botticello, & Sepúlveda, 2005) and adjustment for covariates (Fergusson et al., 2007; Roberts, Roberts, & Chen, 1997), likely contribute to the mixed findings reported in these areas.
The purpose of the present investigation is to determine whether the physical activity trajectory from ages 12-17 differs for boys and girls with and without elevated depressive symptoms. A cohort-sequential, multiple-group (elevated depressive symptoms vs. non-elevated depressive symptoms) design was employed within a latent growth model (LGM) to help shed light on: (a) the relations between level of depressive symptoms and physical activity; (b) whether youth with elevated initial levels of depressive symptoms are more or less at risk for physical activity declines during adolescence compared to those without; and (c) the influences of family, personal, and demographic variables on physical activity trajectories of adolescents with and without elevated depressive symptoms. Hypotheses tested were: (a) average levels (group means) of physical activity significantly decrease throughout this period for youth with and without elevated depressive symptoms; (b) individual physical activity participation varies significantly around group means for both subgroups; (c) adolescents with elevated depressive symptoms participate in less physical activity and have a greater decrease in physical activity over time compared to adolescents without elevated depressive symptoms; (d) girls are less physically active than boys; (e) White youth and those from higher-income families are more active than non-White youth and those from lower-income families, regardless of depressive symptoms; (f) parent physical activity and parental support for physical activity are positively related to physical activity in adolescents with and without elevated depressive symptoms; and (g) adolescents with higher initial BMI and advanced physical maturation are less active than those with lower initial BMI and less-advanced physical maturation, regardless of initial depressive symptom level.
2. Methods
2.1. Participants and procedures
Data for this study were collected from youth residing in a metropolitan area in the Pacific Northwest. As part of a longitudinal cohort-sequential study design, data were collected from three age cohorts (ages 10, 12, and 14). Families having eligible children were randomly recruited from 58 neighborhoods, primarily using a computer-aided telephone interviewing (CATI) system. Recruitment was balanced across seasons. Of eligible families, approximately 68% agreed to participate (Duncan, Strycker, Duncan, & Chaumeton, 2002). Compared to 2000 census data, families in this study were representative of the county from which they were recruited in terms of race (76% vs. 79% White) and family structure (23% vs. 20.3% single-parent families).
Participants in the present research were assessed annually for 4 years at their homes. The youth and a parent completed surveys in the presence of trained research assistants. Participants completed individual surveys in private to enhance confidentiality. For 7 days prior to the survey, youth completed a daily record of physical activities and wore a pedometer to record steps taken each day. In Year 1, youth were paid $25 for completing the assessments with a bonus of $5 if all aspects of the assessment were completed. Parents were paid $15. The payment was increased by $5 each year. Appropriate Institutional Review Board approval for research with human subjects was obtained; informed consent (adult) and assent (youth) were obtained for all participants.
Data included 371 youth. The sample was 50% female; and 76% White, 12% African American, 4% Hispanic, 2% Asian, 2% American Indian, and 4% other or mixed races. Mean age at Time 1 was 12.05 years (SD = 1.63). Proportions (in parentheses) of the sample with annual household incomes within the following ranges were: under $30,000 (19%), $30,000–$49,999 (30%), $50,000–$69,999 (26%), $70,000–$89,999 (13%), and $90,000 and above (12%). Attrition from Times 1–4 was 4.3%.
2.2. Measures
2.2.1. Youth physical activity
Because physical activity is a complex multidimensional behavior, researchers emphasize collecting data from multiple sources (Ainsworth, Montoye, & Leon, 1994; Wood, 2000). The suitability of each methodology depends on the type of research, the age and number of research participants, and pragmatic considerations such as the resources available to the researcher (Sallis & Owen, 1999). The measures of youth physical activity used in this study included survey items and data from electronic motion sensors (pedometers).
Survey items
Three survey items were used based on prior measures (Heath, Pate, & Pratt, 1993; Sallis, Buono, Roby, Micale, & Nelson, 1993). The first two items were based on questions from the Youth Risk Behavior Survey (YRBS) (Brener et al., 2004; Heath et al., 1993). Youth were asked: (a) “On how many of the past 7 days did you exercise or take part in hard physical activities that made you sweat and breathe hard for at least 20 minutes without stopping (such as basketball, jogging, swimming laps, fast bicycling, or similar aerobic activities)?” and (b) “In a typical week, how many days do you take part in any regular physical activity long enough to work up a sweat (heart beats rapidly)?” For both items, responses ranged from 0 to 7 days. Reports in this study compare favorably to those from the 1999 YRBS; for example, hard physical activity at equal to or greater than 3 days a week is 69% in this sample vs. 72% in the YRBS data. The third survey item asked, “Compared to others the same age and sex, how much physical activity do you get?” (1 = “much less than others” to 5 = “much more than others”). This item was taken from a study of physical activity self-reports among children aged 10–16 years (Sallis et al., 1993), in which it was found to be valid and reliable (test-retest r = .93) in this age group. Distributions for the self-report questionnaire items were normal with skewness scores ranging from −.35 to .19 for the three items across all 4 years.
Pedometer
The fourth physical activity measure was a pedometer. Pedometers have been shown to be valid and reliable tools for measuring youth physical activity (Kilanowski, Consalvi, & Epstein, 1999; Vincent & Pangrazi, 2002). At the assessment visit, youth were instructed in how to wear a pedometer and asked to record the number of steps taken each day for 7 days. The Yamax Digiwalker SW-701 (Optimal Health Products and Services, San Antonio, TX; $20 U.S.) was used in this study.
Compliance in filling out the 7-day physical activity record was generally very good, with 99% of participants recording the daily pedometer total for at least 4 of the 7 days (70.3% completed all 7 days). The study protocol was designed to maximize use of the pedometer and recording of the data on a daily record. Along with a pedometer, target youth received two project-logo magnets and were instructed to put the form on the refrigerator or in some other prominent place. Parents were encouraged to help remind children, and reminder phone calls were made to the family 2 days after the first visit. An average steps/day variable was computed by totaling the number of steps and dividing by the number of days. The average daily number of steps was then divided by 10,000 to rescale the values to reduce problems related to variance discrepancies across measures in the structural equation model (Gustafsson, Rosén, & Stahl, 2002). Skewness scores for the pedometer were acceptable, ranging from .83 to 1.33. The survey items and pedometer data were used as indicators of the youth physical activity latent factor.
2.2.2. Depressive symptoms
Youth depressive symptoms were measured using a 10-item version of the well-validated Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977). Respondents were asked to rate (on a scale from 0=”rarely or none of the time” to 3=”most or all of the time”) the extent to which over the past week: “I did not feel like eating; my appetite was poor,” “I felt that I could not shake off the blues even with help from my family and friends,” “I felt that I was just as good as other people,” “I felt depressed,” “My sleep was restless,” “I was happy” (reverse scored), “I felt lonely,” “I felt sad,” “I felt that people disliked me,” and “I could not get `going'.” Scores on the CES-D were derived by summing item values and then dividing this sum by the number of questionnaire items (α = .75). These CES-D scores were split into two groups at the sample median (median = 0.38; M=0.44, SD=0.40) to create groups of adolescents with and without elevated depressive symptoms. We chose the median split to create balanced groups and maximize statistical power. Based on a proration of the 10-item scale to the full 20-item version of the CES-D, the depressive symptom group cutoff score would correspond to a sum score of 10 or greater, which represents mild (or greater) severity of depressive symptoms.
2.2.3. Covariates
Covariates included personal physical factors (BMI, physical development), family influences (parent physical activity, parental support for physical activity), and demographic factors (sex, race, household income). All variables were measured at the first assessment (Time 1), and were included as covariates of the physical activity intercept and slope factors. Change scores from Time 1 to Time 4 (calculated as Time 4 minus Time 1) also were included as covariates of the slope, or trajectory, of youth physical activity. Although measures were available for the covariates at all time points, the use of each individual covariate across time would have resulted in extremely complex and likely infeasible models. An acceptable alternative is to include a change score as a predictor (see Kessler & Greenberg [1981] for more information relating to change scores).
2.2.4. Body mass index
Trained assessors measured height (m) and weight (kg) of participants using calibrated, sensitive scales. BMI was calculated as weight (kg) divided by height (m) squared (kg/m2).
2.2.5. Physical maturation
Physical maturation was assessed via self-report items using shortened versions of the boys' and girls' Pubertal Development scales (Brooks-Gunn, Warren, Rosso, & Gargiulo, 1987; Peterson, Crockett, Richards, & Boxer, 1988). For this study, there were four items for girls and five items for boys. This measure provides an assessment of the child's level of maturation with regard to changes in body proportions (e.g., “growth in height” and “growth in breasts” for girls), facial features (e.g., “growth in body hair,” “skin changes, especially pimples,” and “hair growth on your face” for boys), and, for boys only, “deepening of your voice.” Youth responded on a 4-point scale from (1) “Not yet begun to change” to (4) “This change seems completed.” Internal consistency in this study was α = .80 for girls and α = .79 for boys.
2.2.6. Parent physical activity
One parent per family reported on his/her own and the partner's (if applicable) physical activity. Parents were asked, “In a typical week, how many days do you take part in any regular physical activity long enough to work up a sweat (heart beats rapidly)?” Responses ranged from 0 to 7 days. Where appropriate, a mean score of own and spouse/partner's activity was computed.
2.2.7. Parental support for physical activity
Similar to items used in other studies (Duncan et al., 2005; Sallis et al., 2002), youth were asked the extent to which their parents provided different types of support for physical activity. These included informational and emotional support (“Encourage you to do physical activities,” “Watch you take part in physical activities,” and “Talk with you about your physical activity”) and instrumental support/tangible aid (“Do a physical activity with you” and “Provide transportation so you can go to a place where you can do physical activities”). Questions asked how often during a typical week each parent did these things. Responses were on a 5-point scale ranging from (1) “Never” to (5) “Very often.” The internal consistency of the parental support for physical activity scale was α =.78.
2.2.8. Demographic variables
Demographic variables of youth sex, race, and family income were included in the model to control for their possible effects on youth physical activity over time. Youth sex was coded 1 = male and 0 = female. Because most participants were White, race (self-report) was coded 1 = white and 0 = other races. Parents also reported annual household income, which was divided into 11 categories, the lowest being “under $5,000” and the highest “more than $90,000.” Youth age was also included in the model as part of the multiple-group, cohort-sequential design.
2.3. Analyses
LGM was used in this study to model developmental change (the trajectory) of physical activity among youth ages 12-17 years. LGM has numerous advantages over traditional methods for constructing complex and dynamic models that assess change (Duncan, Duncan, & Strycker, 2006; Nesselroade & Baltes, 1979). This strategy can describe a single individual's developmental trajectory and capture important group statistics in a way that allows the researcher to study development at the group level. LGMs are able to test which predictor variables affect the rate of development. Multivariate or higher-order LGMs allow examination of the degree to which relations among growth factors can be described by common higher-order constructs, and, similar to other structural equation models, permit testing of multiple samples in the same model. Accelerated or cohort-sequential designs also can be incorporated into LGMs.
2.3.1. Cohort-sequential LGM
Given time constraints, concerns about attrition, and the cost of multiple assessments, researchers have long sought ways to maintain the advantages and minimize the disadvantages of the longitudinal design (Duncan et al., 2006). Bell (1953) first introduced the idea of “convergence,” a method that calls for limited repeated measurements of independent age cohorts, resulting in temporally overlapping measurements of the various groups. This technique, which has gained popularity as the “cohort-sequential” (McArdle, 1988) or accelerated design, provides a way to link adjacent segments of limited longitudinal data from different age cohorts to determine the existence of a common developmental trend, or growth curve. The analyses in this study incorporated a cohort-sequential specification (see Duncan et al. [2006] for a complete discussion), combining information from three different age cohorts simultaneously to approximate a long-term longitudinal study from ages 12-17 years.
Because of the overlap between ages, it was possible to test the hypothesis that a common developmental trajectory existed for physical activity from ages 12-17. Each age cohort contributed a different section of the overall curve. Because each cohort represented a different pattern of “missingness” in the overall developmental curve, it was possible to build the complete curve using information from all cohorts simultaneously. The same developmental model was assumed in each cohort, permitting tests of convergence across groups and the feasibility of specifying a common growth trajectory across 6 years.
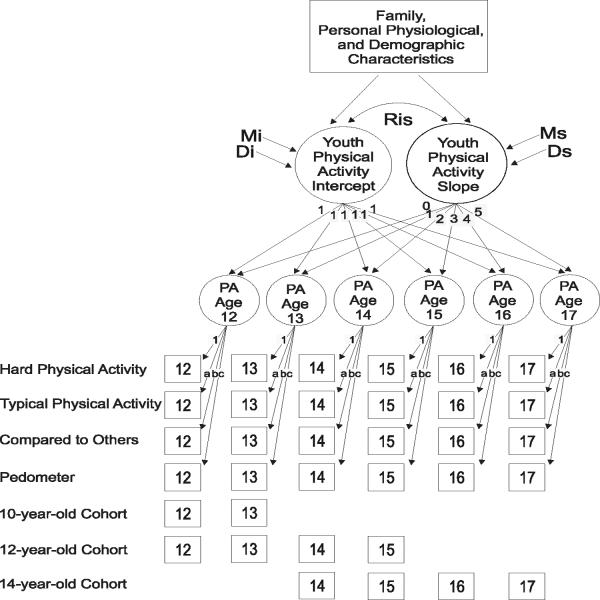
Figure 1 depicts the model tested in this study. As shown in the bottom rows, data were collected in the 12- and 14-year-old cohorts, each measured at four approximately equal yearly intervals. Due to mean and variance issues with the 10-year-olds' data at the first two assessments (ages 10 and 11 years), data for this cohort were used at only two yearly intervals (ages 12 and 13 years). Specifically, data for the 10-year-old cohort at ages 10 and 11 years showed no significant variance in the model, which led to negative error variance estimates for the first two time points and an inadmissible solution for the model. This variance restriction could not be overcome because no other cohort contributed data for these two ages. The same model was assumed in each cohort (using cross-group constraints), allowing for tests of hypotheses concerning convergence across separate groups and the feasibility of specifying a common developmental trajectory.
Figure 1.
Representation of the cohort-sequential curve-of-factors latent growth model.
2.3.2. Higher-order curve-of-factors LGM
LGMs often are used to model growth as a factor of repeated observations of a single variable. In this study, youth physical activity was not represented by a single variable, but as a latent factor reflecting four variables. Therefore, it was necessary to use a multivariate or higher-order LGM to adequately represent change over time in the latent youth physical activity factor.
A curve-of-factors multivariate LGM was used to determine the trajectory of youth physical activity from ages 12-17 years. Within the model, a growth curve was fitted to factor scores representing what the four physical activity measures had in common at each age. That is, within the model the observed variables at each age were factor-analyzed to produce youth physical activity factor scores, which were then used for modeling the physical activity growth curve (McArdle, 1988). Based on prior research, a linear growth model was used to capture the trajectory of physical activity from ages 12-17 years.
In line with structural modeling conventions, squares in Figure 1 denote observed variables and circles denote latent variables. In the figure, the first common, or second-order, factor is labeled Youth Physical Activity Intercept and is a constant for any individual across time. The intercept represents information concerning the mean, represented by Mi, and variance, represented by Di, of the collection of individual intercepts that characterize each individual's growth curve. The second factor, labeled Youth Physical Activity Slope, represents the trend of an individual's trajectory determined by the repeated measures. The second-order slope factor has a mean, Ms, and variance, Ds, across the whole sample. The basis terms (factor loadings) of the intercept are fixed at 1. The basis terms of the slope are fixed (0 to 5) to represent a linear growth trend in physical activity from ages 12-17 years. The slope and intercept are allowed to covary, Ris, shown by the double-headed arrow between the two factors.
In fitting the curve-of-factors LGM, unique covariances for each variable over time were allowed to covary. The curve-of-factors LGM requires a condition of factor pattern invariance where common factor pattern elements must be equal over time. In Figure 1, the variable “Hard Physical Activity” was used as the scaling reference for the first-order common factors (PA Age 12, 13, etc.), and the loadings for “Typical Physical Activity,” “Compared to Others,” and the pedometer data were constrained to be equal across time, as represented by the factor loadings a, b, and c, respectively. These first-order factors were then used as time-varying indicators for the second-order youth physical activity LGM. Detailed rationales for the metric invariance assumptions are provided by Nesselroade (1983). Additional information on the specification of the curve-of-factors LGM can be found in McArdle (1988) and Duncan et al. (2006).
2.3.3. Comparisons across youth with and without elevated depressive symptoms
A multiple-sample framework was used in the LGM analyses to compare youth with and without elevated depressive symptoms. Such multiple-sample analyses permit simultaneous evaluation of developmental hypotheses on multiple populations (e.g., youth with and without elevated depressive symptoms) (Duncan et al., 2006; Muthén & Muthén, 2004). In this study, the analyses tested for significant differences by depressive symptom groups in initial levels of physical activity, in the development of physical activity, and/or in those variables believed to exert effects on the growth parameters. LGM analyses were conducted using Mplus structural equation modeling software, version 6.1 (Muthén & Muthén, Los Angeles, CA, 2010). Program code for the LGM cohort-sequential curve-of-factors model is available from the authors upon request.
3. Results
3.1. Missing data analyses
In the present study, the percent of missing data across all variables ranged from 0% to 13%, with most variables having 5% or less missing data. Thus, to make use of all possible data and reduce the likelihood of biased estimates, imputation of missing data was conducted using an expectation-maximization (EM)-based procedure. The imputed data were then used in the LGM analyses. Means and variances for each of the variables in the model are shown in Table 1.
Table 1.
Means and Standard Deviations of the Variables in the Model
| Mean | SD | |
|---|---|---|
| Physical Activity Variables | ||
| Time 1 hard PA | 3.50 | 1.94 |
| Time 2 hard PA | 3.35 | 2.01 |
| Time 3 hard PA | 3.26 | 1.99 |
| Time 4 hard PA | 2.95 | 2.03 |
| Time 1 typical PA | 3.99 | 1.88 |
| Time 2 typical PA | 3.84 | 1.84 |
| Time 3 typical PA | 3.73 | 1.81 |
| Time 4 typical PA | 3.64 | 1.83 |
| Time 1 PA compared to others | 3.54 | 0.91 |
| Time 2 PA compared to others | 3.52 | 0.96 |
| Time 3 PA compared to others | 3.43 | 0.98 |
| Time 4 PA compared to others | 3.37 | 1.02 |
| Time 1 Pedometer | 1.04 | 0.42 |
| Time 2 Pedometer | 1.02 | 0.45 |
| Time 3 Pedometer | 0.99 | 0.42 |
| Time 4 Pedometer | 0.98 | 0.44 |
| Covanates at Time 1 | ||
| Sex (% female) | 50.1% | |
| Race (% White) | 76% | |
| Household income | 6.74 | 2.51 |
| Body mass index (BMI) | 22.4 | 5.36 |
| Physical maturation | 2.32 | 0.76 |
| Parent PA | 2.49 | 1.63 |
| Parental support for PA | 3.44 | 0.86 |
| Covariates – Change Scores (Time 4 minus Time 1) | ||
| BMI change | 2.03 | 3.26 |
| Parent PA change | −0.15 | 1.09 |
| Parental support for PA change | 0.54 | 1.56 |
3.2. LGM analyses
3.2.1. Unconditional model
A series of LGM models was tested. Analyzed first was a linear unconditional or measurement model (e.g., a model containing no covariates) to determine the viability of the multiple-sample, cohort-sequential, curve-of-factors LGM. Constraints were placed across ages to determine the viability of the cohort-sequential model, and across groups with and without elevated depressive symptoms. The model fit for the linear unconditional model was χ2(334, N = 371) = 493.23, p < .05, Comparative Fit Index (CFI) = .93, and Root Mean Square Error of Approximation (RMSEA) = .05. Values of .90 or greater on the CFI and .05 or less on the RMSEA suggest a model which adequately fits the relationships among the observed data. The observed physical activity variables all loaded significantly on the first-order physical activity age factors. As indicated earlier and shown in Figure 1, the variable “Hard Physical Activity” was used as the scaling reference for the first-order common factors (PA Age 12, 13, etc.) and thus set at “1.” The loadings for the other variables on the first-order age factors were λ = 1.05, t = 20.78 for “Typical Physical Activity,” λ = .41, t = 15.55 for “Compared to Others,” and λ = .11, t = 9.77 for the pedometer data. Loadings for “Typical Physical Activity,” “Compared to Others,” and the pedometer data were constrained to be equal across time. The pedometer variable loaded less significantly than the other variables on the physical activity factor, but its contribution was significant and is associated with a large effect size (partial r =.47).
Significant mean levels existed for the higher-order common factors of the youth physical activity intercept (age 12), Mi = 3.73, t = 35.80, and slope (growth from ages 12–17 years), Ms = −.242, t = −6.45. Individual differences in the higher-order intercept and slope factors also were significant, with variances of Di = 1.79, t = 7.11, and Ds = .13, t = 4.12, respectively. These findings indicate that, at the group level, on average, there was a significant decline (negative linear slope) in physical activity from ages 12–17 years, with significant variation among youth in their initial physical activity at age 12 (intercept) and in their trajectory (slope) from ages 12–17. The correlation between the intercept and slope, Ris, also was significant, −.23, t = −2.96, indicating that higher levels of activity at age 12 were associated with greater decreases from ages 12–17.
Results of the linear unconditional model pointed to a significant depressive symptom group difference in the intercept (Time 1) mean. Results suggested that a significant chi-square decrease would occur if this depressive symptom group constraint were relaxed. Thus, a second unconditional model was analyzed with the constraint relaxed on the intercept mean. The fit for this model was χ2(333, N = 371) = 486.35, p < .05, CFI = .94, and RMSEA = .05. With this constraint relaxed, mean levels of physical activity on the intercept were significant for both groups, but youth without elevated depressive symptoms had significantly higher mean levels of physical activity at age 12, Mi = 3.98, t = 28.51, compared to youth with elevated depressive symptoms, Mi = 3.55, t = 28.82. No other significant differences by depressive symptom status were indicated. Thus, this model represents the final linear unconditional model, with a significant youth physical activity slope mean, Ms = −.25, t = −6.53, and significant variances on the intercept (age 12), Di = 1.76, t = 7.07, and slope (growth from ages 12–17), Ds = .13, t = 4.13. The mean slope score represents an average decrease in physical activity of approximately 18% from ages 12–17 years. In this model, the loadings for the physical activity variables on the first-order age factors remained significant, λ = 1.06, t = 20.65 for “Typical Physical Activity,” λ = .41, t = 15.49 for “Compared to Others,” and λ = .11, t = 9.74 for the pedometer data. The correlation between the intercept and slope, Ris, remained significant, −.23, t = −3.01.
In sum, the results of the linear unconditional model indicated the appropriateness of the curve-of-factors and cohort-sequential model for describing these physical activity data from ages 12–17 years.
3.2.2. Conditional model
The next model tested was a linear conditional model with all the covariates included. Not surprisingly, given the number of variables, the model fit with all covariates was not good, χ2 (779, N = 371) = 1123.34, p < .05, CFI = .87, and RMSEA = .05. Backwards elimination (Pedhazur, 1997) of covariates was used (at a significance level of p < .05) to derive the most parsimonious model among the set of predictors. With this method, all independent variables are initially included in the model, each variable is treated as if it were entered last in the equation, and variables that do not significantly add to the equation are deleted one at a time until all remaining variables in the model are significant. Regardless of significance value, demographic characteristics (sex, race, and family income) were retained in the final model to control for any influence they might have on youth's initial levels and trajectory of physical activity. The final conditional model resulted in the following acceptable fit indices: χ2(599, N = 371) = 862.29, p < .05, CFI = .904, and RMSEA = .048. The covariate effects for youth with and without elevated depressive symptoms are shown in Table 2.
Table 2.
Standardized Regression Coefficients and t-values for Covariates in the final Model
| Intercept (Age 12) | Slope (Growth 12–17 Years) | |||
|---|---|---|---|---|
| Estimate | t Value | Estimate | t Value | |
| Non-Depressive Symptom Group | ||||
| Sex | .632 | 2.66 | .051 | 0.60 |
| Race | −.248 | −0.86 | .064 | 0.65 |
| Income | −.001 | −0.18 | .003 | 1.85 |
| Time 1 parent PA | −.008 | −.112 | .014 | 0.54 |
| Time 1 parental support for PA | .706 | 4.88 | −.060 | −1.41 |
| Parent activity change | NA | NA | .182 | 5.43 |
| Depressive Symptom Group | ||||
| Sex | .680 | 3.00 | .037 | 0.48 |
| Race | .579 | 2.11 | − .216 | − 2.30 |
| Income | .005 | 0.10 | .001 | 0.07 |
| Time 1 parent PA | −.009 | −0.14 | .022 | 0.93 |
| Time 1 parental support for PA | .393 | 3.22 | .043 | 1.13 |
| Parent activity change | NA | NA | .082 | 3.21 |
Note. Bolded entries are significant at p < .05.
There was a significant effect of sex for both depressive symptom groups, with boys having higher initial levels of physical activity than girls across both groups. Race (White vs. non-White) effects were evident only in the elevated depressive symptom group; Whites had higher initial levels of physical activity than non-Whites, but had more of a decrease in physical activity from ages 12–17 years than non-Whites. Similar effects of Time 1 parental support for physical activity were found across the two depressive symptom groups. Greater perceived parental support for physical activity was related to higher initial levels of adolescent physical activity regardless of elevated depressive symptom status. Effects of parental physical activity change also were similar for the depressive symptom groups, indicating that the more parents increased (changed positively) in their own physical activity over time, the less of a decline (or more of an increase) in the physical activity trajectories of their adolescent children. Thus, the results indicate a protective role of parental physical activity and parental support for physical activity on their children's levels and trajectories of physical activity across both depressive symptom groups.
For youth without elevated depressive symptoms, 33% and 23% of the variance in the intercept and slope, respectively, was accounted for by the final model's covariates. For youth with elevated depressive symptoms, 18% and 17% of the variance in the intercept and slope, respectively, was accounted for by the final model's covariates.
4. Discussion
Few studies have examined adolescent physical activity trajectories, especially as a function of level of depressive symptoms. Familial, personal, physiological, and demographic covariates of initial status and change in adolescent physical activity were included, and the model was tested across youth with and without elevated depressive symptoms. As hypothesized and in line with previous research (Jerstad et al., 2010), the results of the study provide evidence of a significant decline in physical activity from ages 12–17 years across all adolescents, regardless of depressive symptoms. Significant variation around the group means also was evident for both depressive symptom groups.
As expected, and supported by previous research (Boone & Leadbeater, 2006), youth without elevated depressive symptoms were significantly more physically active than youth with elevated depressive symptoms initially, which in this study was age 12. Contrary to expectations, however, there were no significant differences between depressive symptom groups in their physical activity trajectories. Both groups, on average, had a similar decreasing trajectory of physical activity from ages 12–17, suggesting that adolescents with elevated depressive symptoms did not appear to have a faster rate of physical activity decline than adolescents without elevated depressive symptoms. The relationship of initial depressive symptoms with lower levels of physical activity supports studies indicating an inverse relationship between adolescent physical activity and depressive symptoms (e.g., Motl et al., 2004). The lack of differences in trajectories for the two groups provides partial support for the Birkeland et al. (2009) study where they found no prospective relations between physical activity and depressive symptoms in adolescents.
Covariates in this study included demographic variables of sex, race (White vs. non-White), and family income; family variables of parent physical activity and parental support for physical activity for the youth's physical activity; and personal physiological variables of BMI and physical maturation. As hypothesized, there was an effect for sex such that boys had significantly higher initial levels of physical activity than girls, regardless of depressive symptom status. This result supports general research on adolescent physical activity showing that boys participate in physical activity more than girls (Suris & Parera, 2005; Whitt-Glover et al., 2009). Race effects were hypothesized regardless of depressive symptom status; however, these were evident only for the group with elevated depressive symptoms. Similar to prior research, White adolescents had higher levels of initial physical activity than did non-White adolescents (Brodersen et al., 2005; Santos et al., 2004). The significant effect for race on the physical activity trajectory also indicates that White youth had more of a decrease in physical activity from ages 12–17 than did non-Whites. This is likely a function of their higher initial physical activity levels, which allows more scale movement opportunity than among those with lower initial levels. Family income did not emerge as a significant covariate.
Research has been limited on the effects of puberty on adolescent physical activity (Bradley et al., 2000). While it appears likely that physical maturation and BMI might influence physical activity levels and trajectories from ages 12–17, these variables were not significant covariates of physical activity for either depressive symptom group in this study. Findings based on Petersen and Taylor's (1989) “Mediated Effects Model of Psychological and Behavioral Adaptation to Puberty” provide a possible explanation as to why physical maturation did not emerge as a significant covariate in the current study. The model posits that the effects of maturation on behavior are mediated by psychological variables and moderated by exogenous or contextual factors (Cumming et al., 2011; Petersen & Taylor, 1989). For example, in a sample of adolescent girls, Cumming et al. (2011) found that the relation between physical maturity and physical activity was mediated by physical self-concept. Thus, it is possible that the effects of personal physiological factors might exert their influence in a more indirect or interactive manner with other variables (e.g., physical self-concept) that were not included in the current model.
The strongest influences on physical activity in this study were parental support for their child's physical activity and parents' own physical activity. As hypothesized, these effects were influential across both depressive symptom groups. Greater parental support for physical activity was related to higher initial levels of physical activity (age 12). Similarly, effects of parents' physical activity change were found across both groups: Greater increases in parental physical activity were related to less of a decline in the physical activity of their children from ages 12–17. These effects were somewhat stronger in the elevated depressive symptom group, but significant in both groups. These findings support prior research emphasizing the importance of parental support and parents' own activity in influencing adolescent physical activity (Beets et al., 2006; Roesch et al., 2009; Sallis et al., 2002; Welk et al., 2003). Results also point to an important, possibly protective, role of parental physical activity and parental support for physical activity on physical activity trajectories across adolescents regardless of depressive symptoms. Given that increasing research (e.g., Jerstad et al., 2010) suggests a possible intervening role of physical activity on adolescent depressive symptoms, these findings highlight the influence of family involvement in promoting physical activity among adolescents with elevated depressive symptoms.
This study has limitations and strengths. The research was conducted in one metropolitan area with a predominantly White sample, restricting the ability to make group comparisons by race. The complexity of the model necessitated including sex as a covariate rather than as part of the multiple-sample framework; thus, we were unable to determine whether trajectories differed for boys and girls as a function of depressive symptoms. The physical activity and depression measures have limitations. Measurement of youth physical activity is difficult, with no measure being ideal for every situation. The assessment of depressive symptoms in this study also warrants comment. The CES-D, originally developed for epidemiologic surveys, is one of the most widely used screening measures of depressive symptomatology used in research (Eaton, Smith, Ybarra, Muntaner, & Tien, 2004), and has been found to adequately screen for major depressive episodes among adolescents (Roberts, Lewinsohn, & Seeley, 1991). Strengths of this measure include its ease of use, substantial research base, and strong psychometric qualities, including its sensitivity and specificity for detecting depression from other forms of psychopathology (Eaton et al., 2004). There are also limitations of the CES-D. It is not intended to be a diagnostic tool for assessing major depressive episodes, scale items are not intended to assess Diagnostic and Statistical Manual of Mental Disorders-defined symptoms of depression, and it is relatively insensitive to changes in level of depression among those diagnosed with a major depressive disorder (Eaton et al., 2004).
Because results of the study are affected by the physical activity and depression measures used, it must be noted that other studies with different measures may yield different results. The covariates included in the study represent only a small number of possible potential influences on adolescent physical activity and depressive symptoms. Another limitation is the lack of variance in physical activity at ages 10 and 11, which necessitated the omission of these data from the LGM. Future studies with different samples are needed to determine whether trajectories of physical activity for youth can be modeled for younger age groups as a function of depressive symptoms.
Major strengths of the study are the longitudinal nature of the data and design, the use of LGM and cohort-sequential modeling to test the trajectory of physical activity from ages 12–17 with only four annual assessments, and a multiple-group model specification to determine effects of depressive symptoms. The longitudinal nature of the study addresses a critical need for data on patterns of youth physical activity among individuals with and without depressive symptoms, and how these patterns change over time. The inclusion of personal physiological, family, and demographic covariates are strengths, as are the use of a randomly recruited sample, self- and parent-report data, and the utilization of different data methods (survey items and pedometer data) to document physical activity.
The latent variable approach is a powerful technique for the operationalization of physical activity, as it offers an efficient and appropriate way to combine several physical activity variables into one factor for analysis. There are many advantages of LGM, including its flexibility, practicality, and value for modeling developmental processes, and its ability to identify important predictors and outcomes of change. Unfortunately, to date LGM has been underutilized in studies of physical activity and depressive symptoms and disorders. Future studies are encouraged to use procedures such as LGM to examine the etiology and development of youth physical activity from childhood through adolescence to better understand the relationship between physical activity and depressive symptoms, and other mental health outcomes in general.
5. Conclusions
The present findings indicate that physical activity declines from ages 12–17 regardless of level of depressive symptoms and that girls are less active than boys. Parental support for their child's physical activity and parents' own physical activity appear to be strong influences of adolescent physical activity for adolescents with and without elevated depressive symptoms. Results highlight the importance of family involvement in promoting physical activity among adolescents. These findings have practical implications for those seeking to design effective interventions for maintaining physical activity and ameliorating depressive symptoms throughout childhood, adolescence, and the life span.
Highlights
This was one of few studies to relate adolescent depression and physical activity.
Physical activity declined from ages 12-17 regardless of depression levels.
Increases in parent physical activity related to less decline in adolescent physical activity.
Adolescents with more depressive symptoms had lower initial physical activity.
Parental support of physical activity was associated with physical activity in youth.
Acknowledgments
This research was supported by Grant HD35873 from the National Institute of Child Health and Human Development. Preparation of this manuscript was supported in part by the Institute of Education Sciences, U.S. Department of Education, through Grant R324A090111 to the Oregon Research Institute. The opinions expressed are those of the authors and do not represent views of the institutes or the U.S. Department of Education.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainsworth BE, Montoye HJ, Leon AS. Methods of assessing physical activity during leisure and work. In: Bouchard C, Shepard RJ, Stevens T, editors. Physical activity, fitness, and health: International proceedings and consensus statement. Human Kinetics; Champaign, IL: 1994. pp. 146–159. [Google Scholar]
- Baker BL, Birch LL, Trost SG, Davison KK. Advanced pubertal status at age 11 and lower physical activity in adolescent girls. Journal of Pediatrics. 2007;151:488–493. doi: 10.1016/j.jpeds.2007.04.017. doi:10.1016/j.jpeds.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beets MW, Vogel R, Forlaw L, Pitetti KH, Cardinal BJ. Social support and youth physical activity: The role of provider and type. American Journal of Health Behavior. 2006;30:278–289. doi: 10.5555/ajhb.2006.30.3.278. doi:10.5993/AJHB.30.3.6. [DOI] [PubMed] [Google Scholar]
- Bell RQ. Convergence: An accelerated longitudinal approach. Child Development. 1953;24:145–152. doi:10.2307/1126345. [PubMed] [Google Scholar]
- Berkey CS, Rockett HRH, Field AE, Gillman MW, Frazier AL, Camargo CA, Jr., et al. Activity, dietary intake, and weight changes in a longitudinal study of preadolescent and adolescent boys and girls. Pediatrics. 2000;105:56. doi: 10.1542/peds.105.4.e56. doi:10.1542/peds.105.4.e56. [DOI] [PubMed] [Google Scholar]
- Birkeland MS, Torsheim T, Wold B. A longitudinal study of the relationship between leisure-time physical activity and depressed mood among adolescents. Psychology of Sport and Exercise. 2009;10:25–34. doi:10.1016/j.psychsport.2008.01.005. [Google Scholar]
- Boone E, Leadbeater B. Game on: Diminishing risks for depressive symptoms in early adolescence through positive involvement in team sports. Journal of Research on Adolescence. 2006 Mar;16:79–90. doi:10.1111/j.1532-7795.2006.00122.x. [Google Scholar]
- Bradley CB, McMurray RG, Harrell JS, Deng S. Changes in common activities of 3rd through 10th graders: The CHIC study. Medicine and Science in Sports and Exercise. 2000;32:2071–2078. doi: 10.1097/00005768-200012000-00017. doi:10.1097/00005768-200012000-00017. [DOI] [PubMed] [Google Scholar]
- Brodersen NH, Steptoe A, Williamson S, Wardle J. Sociodemographic, developmental, environmental, and psychological correlates of physical activity and sedentary behavior at age 11 to 12. Annals of Behavioral Medicine. 2005;29:2–11. doi: 10.1207/s15324796abm2901_2. doi:10.1207/s15324796abm2901_2. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren M, Rosso J, Gargiulo J. Validity of self-report measures of girls' pubertal status. Child Development. 1987;58:829–841. doi:10.2307/1130220. [PubMed] [Google Scholar]
- Cortese S, Falissard B, Angriman M, Pigaiani Y, Banzato C, Bogoni G, et al. The relationship between body size and depression symptoms in adolescents. Journal of Pediatrics. 2009;154:86–90. doi: 10.1016/j.jpeds.2008.07.040. doi:10.1016/j.jpeds.2008.07.040. [DOI] [PubMed] [Google Scholar]
- Crews DJ, Lochbaum ML, Landers DM. Exercise effects on psychological well-being and academic achievement in low-income Hispanic children. Perceptual and Motor Skills. 2004;98:319–324. doi: 10.2466/pms.98.1.319-324. doi:10.2466/pms.98.1.319-324. [DOI] [PubMed] [Google Scholar]
- Cui M, Conger RD, Lorenz FO. Predicting change in adolescent adjustment from change in marital problems. Developmental Psychology. 2005;41:812–823. doi: 10.1037/0012-1649.41.5.812. doi:10.1037/0012-1649.41.5.812. [DOI] [PubMed] [Google Scholar]
- Cumming SP, Standage M, Loney T, Gammon C, Neville H, Sherar LB, Malina RM. The mediating role of physical self-concept on relations between biological maturity status and physical activity in adolescent females. Journal of Adolescence. 2011;34:465–473. doi: 10.1016/j.adolescence.2010.06.006. doi:10.1016/j.adolescence.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Harvey AG. Sleep in children and adolescents with behavioral and emotional disorders. In: Jenni OG, Carskadon MA, editors. Sleep medicine clinics. no. 3. vol. 2. W. B. Saunders; Philadelphia, PA: 2007. pp. 501–512. [Google Scholar]
- Davison KK, Werder JL, Trost SG, Baker BL, Birch LL. Why are early maturing girls less active? Links between pubertal development, psychological well–being, and physical activity among girls at ages 11 and 13. Social Science & Medicine. 2007;64:2391–2404. doi: 10.1016/j.socscimed.2007.02.033. doi:10.1016/j.socscimed.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson SJ, Ronan KR. The effects of sports participation on young adolescents' emotional well-being. Adolescence. 2006;41:369–389. [PubMed] [Google Scholar]
- Duncan SC, Duncan TE, Strycker LA. Sources and types of social support in youth physical activity. Health Psychology. 2005;24:3–10. doi: 10.1037/0278-6133.24.1.3. doi:10.1037/0278-6133.24.1.3. [DOI] [PubMed] [Google Scholar]
- Duncan SC, Strycker LA, Duncan TE, Chaumeton NR. Telephone recruitment of a random stratified youth sample for a physical activity study. Journal of Sport and Exercise Psychology. 2002;24:347–358. doi: 10.1016/j.jaging.2004.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC, Strycker LA. An introduction to latent variable growth curve modeling: Concepts, issues, and applications: Revised edition. Lawrence Erlbaum Associates; Mahwah, NJ: 2006. [Google Scholar]
- Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A. Center for Epidemiologic Studies Depression Scale: Review and Revision (CESD and CESD-R) In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment (3rd ed.): Volume 3. Instruments for adults. Erlbaum; Mahwah, NJ: 2004. pp. 363–377. [Google Scholar]
- Fergusson DM, Boden JM, Horwood J. Exposure to single parenthood in childhood and later mental health, educational, economic, and criminal behavior outcomes. Archives of General Psychiatry. 2007;64:1089–1095. doi: 10.1001/archpsyc.64.9.1089. doi:10.1001/archpsyc.64.9.1089. [DOI] [PubMed] [Google Scholar]
- Garber J, Keiley MK, Martin NC. Developmental trajectories of adolescents' depressive symptoms: Predictors of change. Journal of Consulting and Clinical Psychology. 2002;70:79–95. doi: 10.1037//0022-006x.70.1.79. doi:10.1037/0022-006X.70.1.79. [DOI] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1768–1776. doi: 10.1097/00004583-199712000-00026. doi:10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Goodman E, Slap GB, Huang B. The public health impact of socioeconomic status on adolescent depression and obesity. American Journal of Public Health. 2003;93:1844–1850. doi: 10.2105/ajph.93.11.1844. doi:10.2105/AJPH.93.11.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson J, Rosén M, Stahl PA. STREAMS user's guide (Version 2.5) [Computer software and manual] MultiariateWare; Mölndal, Sweden: 2002. [Google Scholar]
- Hammen C. Adolescent depression: Stressful interpersonal contexts and risk for recurrence. Current Directions in Psychological Science. 2009;18:2000–2004. doi: 10.1111/j.1467-8721.2009.01636.x. doi:10.1111/j.1467-8721.2009.01636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL. Adolescent depression: Description, causes, and interventions. Epilepsy Behavior. 2006;8:102–114. doi: 10.1016/j.yebeh.2005.10.012. doi:10.1016/j.yebeh.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Heath GW, Pate RR, Pratt M. Measuring physical activity among adolescents. Public Health Reports. 1993;108:42–46. [PMC free article] [PubMed] [Google Scholar]
- Jerstad SJ, Boutelle KN, Ness KK, Stice E. Prospective reciprocal relations between physical activity and depression in female adolescents: A longitudinal study. Journal of Clinical and Consulting Psychology. 2010;78:268–272. doi: 10.1037/a0018793. doi:10.1037/a0018793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltiala-Heino R, Rimpelä M, Rantanen P, Laippala P. Adolescent depression: The role of discontinuities in life course and social support. Journal of Affective Disorders. 2001;64:155–166. doi: 10.1016/s0165-0327(00)00233-0. doi:10.1016/S0165-0327(00)00233-0. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Greenberg DF. Linear panel analysis: Models of quantitative change. Academic Press; New York: 1981. [Google Scholar]
- Kilanowski CK, Consalvi AR, Epstein LH. Validation of an electronic pedometer for measurement of physical activity in children. Pediatric Exercise Science. 1999;11:63–68. [Google Scholar]
- Kimm SYS, Glynn NW, Kriska AM, Fitzgerald SL, Aaron DJ, Similo SL, et al. Longitudinal changes in physical activity in a biracial cohort during adolescence. Medicine and Science in Sports and Exercise. 2000;32:1445–1454. doi: 10.1097/00005768-200008000-00013. doi:10.1097/00005768-200008000-00013. [DOI] [PubMed] [Google Scholar]
- Klein DN, Torpey DC, Bufferd SJ, Dyson MW. Depressive disorders. In: Beauchaine TP, Hinshaw SP, editors. Child and adolescent psychopathology. Wiley; Hoboken, NJ: 2008. pp. 477–509. [Google Scholar]
- Larun L, Nordheim LV, Ekeland E, Hagen KB, Heian F. Cochrane Database of Systematic Reviews. Cochrane AN: 2009. Exercise in prevention and treatment of anxiety and depression among children and young people; p. CD004691. [DOI] [PubMed] [Google Scholar]
- Lau JFY, Rijsdijk F, Gregory AM, McGuffin P, Eley TC. Pathways to childhood depressive symptoms: The role of social, cognitive, and genetic risk factors. Developmental Psychology. 2007;43:1402–1414. doi: 10.1037/0012-1649.43.6.1402. doi:10.1037/0012-1649.43.6.1402. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Gotlib IH, Seeley JR. Depression-related psychosocial variables: Are they specific to depression in adolescents? Journal of Abnormal Psychology. 1997;106:365–375. doi: 10.1037//0021-843x.106.3.365. doi:10.1037/0021-843X.106.3.365. [DOI] [PubMed] [Google Scholar]
- Lotan M, Merrick J, Carmeli E. Physical activity in adolescence. A review with clinical suggestions. International Journal of Adolescent Medicine and Health. 2004;16:13–21. doi: 10.1515/ijamh.2005.17.1.13. [DOI] [PubMed] [Google Scholar]
- Maxwell MA, Cole DA. Weight change and appetite disturbance as symptoms of adolescent depression: Toward an integrative biopsychosocial model. Clinical Psychology Review. 2009;29:260–273. doi: 10.1016/j.cpr.2009.01.007. doi:10.1016/j.cpr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Dynamic but structural equation modeling of repeated measures data. In: Cattel RB, Nesselroade J, J., editors. Handbook of multivariate experimental psychology. 2nd edition Plenum Press; New York: 1988. pp. 561–614. [Google Scholar]
- McCleary L, Sanford M. Parental expressed emotion in depressed adolescents: Prediction of clinical course and relationship to comorbid disorders and social functioning. Journal of Child Psychology and Psychiatry. 2002;43:587–595. doi: 10.1111/1469-7610.00048. doi:10.1111/1469-7610.00048. [DOI] [PubMed] [Google Scholar]
- Motl WR, Birnbaum AS, Kubik MY, Dishman RK. Naturally occurring changes in physical activity are inversely related to depressive symptoms during early adolescence. Psychosomatic Medicine. 2004;6:336–342. doi: 10.1097/01.psy.0000126205.35683.0a. doi:10.1097/01.psy.0000126205.35683.0a. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus: The comprehensive modeling program for applied researchers. User's guide. 3rd ed. Muthén & Muthén; Los Angeles: 2004. [Google Scholar]
- Negriff S, Susman EJ. Pubertal timing, depression, and externalizing problems: A framework, review, and examination of gender differences. Journal of Research on Adolescence. 2011;21:717–746. doi:10.1111/j.1532-7795.2010.00708.x. [Google Scholar]
- Nesselroade JR. Temporal selection and factor invariance in the study of development and change. Life-Span Development and Behavior. 1983;5:59–87. [Google Scholar]
- Nesselroade JR, Baltes PB. Longitudinal research in the study of behavior and devlopment. Academic Press; New York: 1979. [Google Scholar]
- Paikoff RL, Brooks-Gunn J, Warren MP. Effects of girls' hormonal status on depressive and aggressive symptoms over the course of one year. Journal of Youth and Adolescence. 1991;20:191–215. doi: 10.1007/BF01537608. doi:10.1007/BF01537608. [DOI] [PubMed] [Google Scholar]
- Patten CA, Gillin JC, Farkas AJ, Gilpin EA, Berry CC, Pierce JP. Depressive symptoms in California adolescents: Family structure and parental support. Journal of Adolescent Health. 1997;20:271–278. doi: 10.1016/S1054-139X(96)00170-X. doi:10.1016/S1054-139X(96)00170-X. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Compas BE, Brooks-Gunn J, Stemmler M, Ey S, Grant KE. Depression in adolescence. American Psychologist. 1993;48:155–168. doi: 10.1037//0003-066x.48.2.155. doi:10.1037/0003-066X.48.2.155. [DOI] [PubMed] [Google Scholar]
- Peterson AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. doi:10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Taylor B. The biological approach to adolescence: Biological change and psychological adaptation. In: Adelsen J, editor. Handbook of adolescent psychology. Wiley-Interscience; New York: 1980. [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Roberts RE, Lewinsohn PM, Seeley JR. Screening for adolescent depression: A comparison of depression scales. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:58–66. doi: 10.1097/00004583-199101000-00009. doi:10.1097/00004583-199101000-00009. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Chen YR. Ethnocultural differences in prevalence of adolescent depression. American Journal of Community Psychology. 1997;25:95–110. doi: 10.1023/a:1024649925737. doi:10.1023/A:1024649925737. [DOI] [PubMed] [Google Scholar]
- Roesch SC, Norman GJ, Adams MA, Kerr J, Sallis JF, Ryan S, Calfas KJ, Patrick K. Latent growth curve modeling of adolescent physical activity: Testing parallel process and mediation models. Journal of Health Psychology. 2009;14:313–325. doi: 10.1177/1359105308100216. doi:10.1177/1359105308100216. [DOI] [PubMed] [Google Scholar]
- Rohde P, Beevers CG, Stice E, O'Neil K. Major and minor depression in female adolescents: Onset, course, symptom presentation, and demographic associations. Journal of Clinical Psychology. 2009;65:1339–1349. doi: 10.1002/jclp.20629. doi:10.1002/jclp.20629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton JL, Forcier M, Schectman RM. Epidemiology of depressive symptoms in the National Longitudinal Study of Adolescent Health. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:199–205. doi: 10.1097/00004583-200202000-00014. doi:10.1097/00004583-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Buono MJ, Roby JJ, Micale FG, Nelson JA. Seven-day recall and other physical activity self-reports in children and adolescents. Medicine and Science in Sports and Exercise. 1993;25:99–108. doi: 10.1249/00005768-199301000-00014. doi:10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Owen N. Physical activity and behavioral medicine. Sage; Thousand Oaks, CA: 1999. [Google Scholar]
- Sallis JF, Taylor WC, Dowda M, Freedson PS, Pate RR. Correlates of vigorous physical activity for children in grades 1 through 12: Comparing parent-reported and objectively measured physical activity. Pediatric Exercise Science. 2002;14:30–44. [Google Scholar]
- Santos MP, Esculcas C, Mota J. The relationship between socioeconomic status and adolescents' organized and nonorganized physical activities. Pediatric Exercise Science. 2004;16:210–218. [Google Scholar]
- Seeley JR, Lewinsohn PM. Epidemiology of mood disorders during adolescence: Implications for lifetime risk. In: Allen NB, Sheeber LB, editors. Adolescent emotional development and the emergence of depressive disorders. Cambridge University Press; Cambridge UK: 2008. pp. 33–55. [Google Scholar]
- Seeley JR, Stice E, Rohde P. Screening for depression prevention: Identifying adolescent girls at high risk for future depression. Journal of Abnormal Psychology. 2009;118:161–170. doi: 10.1037/a0014741. doi:10.1037/a0014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeber LB, Davis B, Leve C, Hops H, Tildesley E. Adolescents' relationships with their mothers and fathers: Associations with depressive disorder and subdiagnostic symptomatology. Journal of Abnormal Psychology. 2007;116:144–154. doi: 10.1037/0021-843X.116.1.144. doi:10.1037/0021-843X.116.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Hayward C, Cameron RP, Killen JD, Taylor CB. Body-image and eating disturbance predict onset of depression among female adolescents: A longitudinal study. Journal of Abnormal Psychology. 2000;109:438–444. doi:10.1037/0021-843X.109.3.438. [PubMed] [Google Scholar]
- Suris J, Parera N. Sex, drugs and chronic illness: Health behaviours among chronically ill youth. European Journal of Public Health. 2005;15:484–488. doi: 10.1093/eurpub/cki001. doi:10.1093/eurpub/cki001. [DOI] [PubMed] [Google Scholar]
- Tomson LM, Pangrazi RP, Friedman G, Hutchison N. Childhood depressive symptoms, physical activity, and health related fitness. Journal of Sport and Exercise Psychology. 2003;25:419–439. [Google Scholar]
- Vincent SD, Pangrazi RP. Does reactivity exist in children when measuring activity levels with pedometers? Pediatric Exercise Science. 2002;14:56–63. [Google Scholar]
- Waschbusch DA, Sellers DP, LeBlanc M, Kelley ML. Helpless attributions and depression in adolescents: the roles of anxiety, event valence, and demographics. Journal of Adolescence. 2003;26:169–183. doi: 10.1016/s0140-1971(02)00134-3. doi:10.1016/S0140-1971(02)00134-3. [DOI] [PubMed] [Google Scholar]
- Welk GJ, Wood K, Morss G. Parental influences on physical activity in children: An exploration of potential mechanisms. Pediatric Exercise Science. 2009;15:19–33. [Google Scholar]
- Whitt-Glover MC, Taylor WC, Floyd MF, Yore MM, Yancey AK, Matthews CE. Disparities in physical activity and sedentary behaviors among US children and adolescents: Prevalence, correlates, and intervention implications. Journal of Public Health Policy. 2009;30:S309–S334. doi: 10.1057/jphp.2008.46. doi:10.1057/jphp.2008.46. [DOI] [PubMed] [Google Scholar]
- Wight RG, Aneshensel CS, Botticello AL, Sepúlveda JE. A multilevel analysis of ethnic variation in depressive symptoms among adolescents in the United States. Social Science & Medicine. 2005;60:2073–2084. doi: 10.1016/j.socscimed.2004.08.065. doi:10.1016/j.socscimed.2004.08.065. [DOI] [PubMed] [Google Scholar]
- Wood TM. Issues and future directions in assessing physical activity: An introduction to the conference proceedings. Research Quarterly for Exercise and Sport. 2000;71:ii. doi: 10.1080/02701367.2000.11082779. [DOI] [PubMed] [Google Scholar]