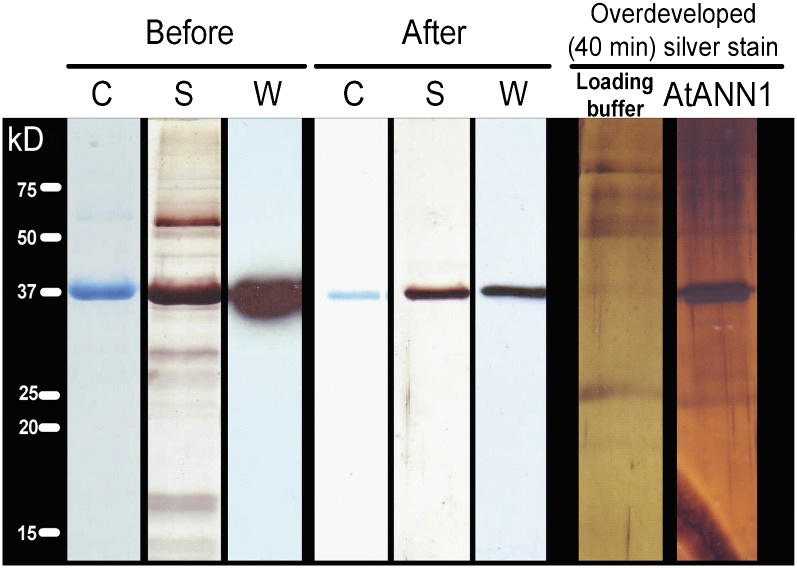
Figure 6.
Purification of Recombinant ANN1.
ANN1 was expressed in S. cerevisiae, and the protein was purified by three cycles of Ca2+-dependent binding to asolectin liposomes (Before) followed by size exclusion chromatography (After). The gel was either colloidal Coomassie blue stained (C), silver stained (S), or used to perform immunoblot analysis (W) to probe for ANN1 using anti-ANN1 peptide antibody. ANN1 is 37 kD. Overdeveloping the silver-stained gel revealed low-level contaminants that were also present in the buffer, including a predominant band at ∼60 kD, which was also found when using S. cerevisiae to produce recombinant mammalian annexins. ANN1 was not detected in preparations from an empty vector control.