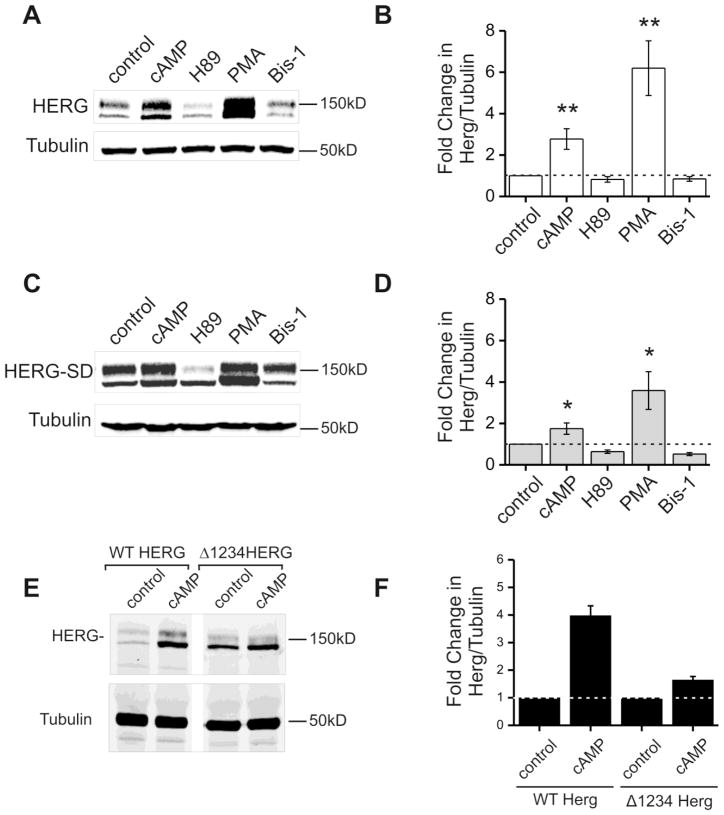
Figure 1. Kinase stimulation and inhibition modulate protein abundance of HERG-WT and HERG-SD.
Parts A and C are representative immunoblots from HEK-HERG wild type and HEK-HERG-SD phosphomimetic mutant stably transfected cells after 24 hour treatment with control (DMSO), CPT-cAMP, H89, PMA, and bisindolylmaleimide-1 (bis-1). HERG appears as a doublet at 135 and 155 kD and tubulin was used as the loading control. Parts B and D show quantification and summary data for the experiment. In part B, cAMP and PMA treatment cause a three- and sixfold increase in wild type HERG protein abundance when normalized to tubulin. H89 and Bis-1 show slight decreases in HERG abundance. Part D shows that HERG-SD the phosphomimetic mutant increases in protein abundance upon both PKA and PKC activation. CPT-cAMP treatment results in a two-fold increase in HERG-SD while PMA causes a four-fold increase. Drug treatment samples were compared to the control, n=5 and * p-value < 0.05, ** p-value < 0.01. Part E is an immunoblot showing lysate from stably expressing HEK-HERG Δ1234 after treatment with control DMSO or CPT-cAMP. Lanes 1 and 2 show blots from cells expressing WT HERG and lanes 3 and 4 show from cells expressing Δ1234HERG. Tubulin was used as the loading control. Part F shows quantification of the fold change in WT HERG and Δ1234 HERG protein abundance normalized to tubulin where the DMSO controls were normalized to 1 (n=2).