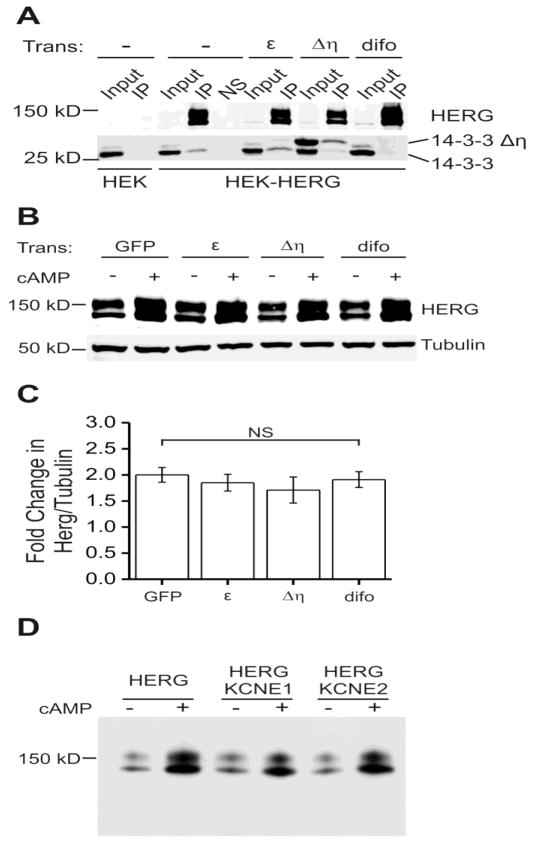
Figure 7. 14-3-3 and KCNE protein binding do not affect the cAMP-induced increase in HERG abundance.
Part A shows immuno-precipitations (IP) from blank HEK or stably expressing HEK-HERG cells with additional transient transfection. Transient transfections were with 14-3-3ε, 14-3-3Δη (dominant negative mutant), and difopein (peptide blocker). The first two lanes of the top panel show a negative control, immunoprecipitation in HEK cells that do not express HERG. Top panel shows input lanes and IP lanes, where HERG is enriched in the IP, and another negative control NS which is mock immunoprecipitation with non-specific IgG. Lower panel shows 14-3-3 isoforms as two distinct bands which co-immunoprecipitated with HERG. The dominant negative mutant 14-3-3Δη migrates at a higher molecular weight. Transient transfection with 14-3-3Δη decreased the amount of endogenous 14-3-3 (lower band) that co-immunoprecipitated with HERG. Transient transfection with difopein abolished the HERG and 14-3-3 binding and thus 14-3-3 is absent in that lane. Part B shows an immunoblot of stably expressing HEK-HERG cells with various transient transfections and 24 hour CPT-cAMP treatment. Transient transfections are with GFP, 14-3-3ε, 14-3-3Δη, and difopein. Upper panel shows HERG protein abundance increases with CPT-cAMP treatment under all transfection conditions. The lower panel shows tubulin as the loading control. Part C shows quantification and summary data for the experiment in part B. The fold changes in HERG normalized to tubulin are similar for all the transient transfection conditions. The differences between GFP transfected and 14-3-3ε, 14-3-3Δη, or difopein transfected groups are not significant, n= 8–27. Panel D shows immunoblots of HEK_HERG cells transiently transfected with GFP (HERG), mink (KCNE1) or MiRP1 (KCNE2) at baseline and after 24-hour stimulation with CPT-cAMP. The cAMP-dependent induction of HERG was not significantly altered by the co-expression of the KCNE family of interacting proteins.