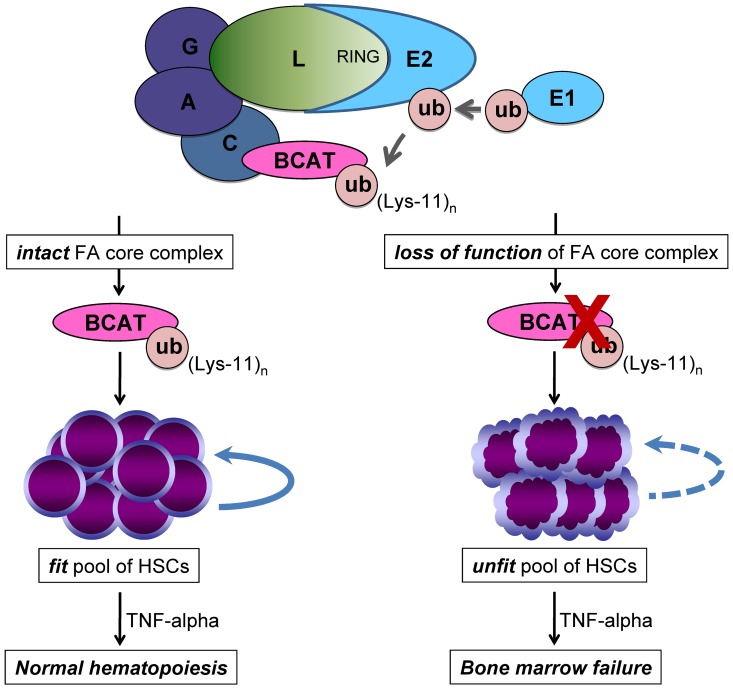
Figure 7.
Proposed model based on our finding that FANCL ubiquitinates β-catenin and enhances its nuclear function. Here we show that FANCL ubiquitinates β-catenin with mixed ubiquitin chain extension involving lysine-11 and this small pool of β-catenin is not targeted for ubiquitin-proteosome degradation but has enhanced activity at Wnt-responsive elements. We propose that the loss-of-function of the FA core complex, specifically FANCL, leads to a reduced pool of active β-catenin modified by lysine-11 ubiquitin chain extension. As a result, there is diminished Wnt/β-catenin signaling in FA HSCs. This molecular defect leads to reduced regenerative capacity and self-renewal of FA HSCs and defines an unfit pool of HSCs susceptible to malignant clonal evolution, especially in the presence of a selective pressure such as TNF-α.24,62