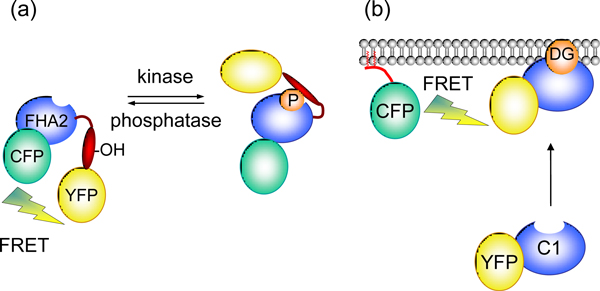
Figure 1.
The architecture of kinase activity reporters. (a) Illustration of the modular architecture used for BKAR, CKAR, and DKAR. The reporter comprises cyan and yellow variants of GFP (CFP and YFP) flanking the phospho-threonine-binding FHA2 domain from the checkpoint kinase rad53 (blue) fused to a substrate sequence (red) for the relevant kinase by a flexible linker. Phosphorylation of the substrate segment causes an intramolecular rearrangement resulting from binding of the FHA2 domain to the phosphorylated substrate sequence (phosphate represented by orange circle). This conformational change alters the amount of FRET from CFP to YFP. The conformational change is readily reversed by dephosphorylation of the substrate segment by cellular phosphatases. The FRET change allows ratiometric measurement of CKAR phosphorylation in cells, revealing the dynamic balance between kinase and phosphatase activities in real time. Note that for other reporters (for example, AKAR, which uses a different phosphopeptide binding module) phosphorylation results in an increase in FRET. (b) A variation of this theme depicting intermolecular FRET used to measure the generation of diacylglycerol (DG) at any membrane of interest. The donor is CFP fused to a targeting sequence for the membrane of interest and the acceptor is the C1 domain, a diacylglycerol-sensing module, fused to YFP.