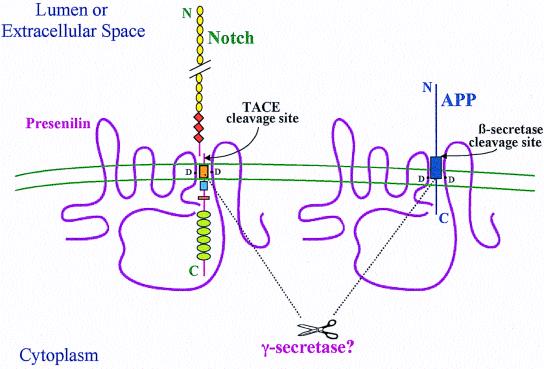
Figure 1.
Hypothetical model of the role of PS in Notch and APP processing based on current information. The diagram shows the predicted eight-transmembrane (TM) domain topology of PS, which occurs principally as a cleaved heterodimer. Some Notch and APP molecules form complexes with PS. Two aspartates (D), one in TM6 and one in TM7 of PS, are required for the cleavages of Notch and APP within their TM domains, and these aspartates may align with each other and with the respective sites of cleavage in the two substrates. It is unknown whether PS directly effects these cleavages or whether a still unidentified aspartyl protease (γ-secretase) present in the complexes does so. PS-mediated proteolysis is preceded by ectodomain shedding caused by tumor necrosis factor α-converting enzyme (TACE) for Notch and β-secretase for APP. Several motifs are depicted in Notch: epidermal growth factor-like repeats (yellow circles), LNG repeats (orange diamonds), a single TM (orange box), the RAM23 domain (blue square), a nuclear localization sequence (red rectangle), and six cdc10/ankyrin repeats (green ovals). After the putative intramembranous cleavage mediated by PS, the Notch intracellular domain is released to the nucleus to activate transcription of target genes. APP contains the Aβ region (dark blue box), which is released into the lumen after sequential cleavages of APP by β-secretase and then γ-secretase/PS. The fate of the APP intracellular domain is unknown.