Abstract
The embryonic foregut of the mouse embryo is lined by a layer of endoderm cells whose architecture changes during development. The transition from a squamous to columnar epithelial morphology is accompanied by the upregulation of an atypical Rho GTPase, Rhou. Subsequently, multi-layering of the epithelium at the site of organ bud formation is associated with the downregulation of Rhou. Rho-related small GTPases are known to play multiple roles in establishing and maintaining epithelial polarity, cytoskeletal organization, morphogenesis and differentiation of epithelial tissues, but their role in the early development of the endoderm in mammals is largely unexplored. Our recent study has shown that Rhou is required for maintaining F-actin polarization, epithelial morphogenesis and differentiation of the endoderm. Rhou expression responds to canonical WNT signaling and its activity influences the cytoskeletal organization and differentiation of endodermal cells, possibly via activation of JNK-mediated pathways. In this context, Rhou provides a possible link between β-catenin dependent WNT signaling and cellular processes normally associated with WNT/PCP pathways.
Keywords: cytoskeleton, differentiation, embryo, endoderm, epithelium, organogenesis, polarity
Introduction
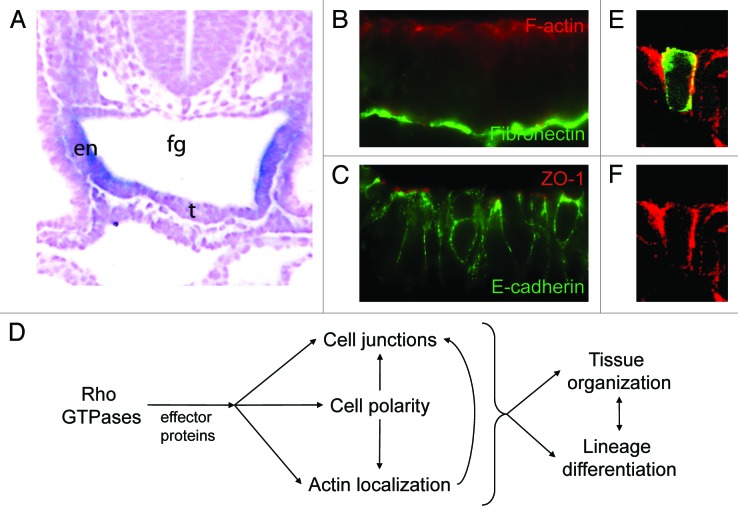
Many of the internal organs of the body, including the liver, pancreas and thyroid, as well as the epithelial linings of the respiratory and digestive tract are derived from the definitive endoderm, which together with the ectoderm and mesoderm constitute the three primary embryonic germ layers. In mouse embryos, the endoderm is initially a monolayered epithelium that lines the primitive gut tube (Fig. 1A). It is polarized in an apical-basal orientation, with actin microfilaments concentrated in a shroud beneath the apical (luminal) surface of the cell (Fig. 1B) and with actin-rich microvilli on its apical surface. Tight junctions (marked by expression of ZO-1) form at the apical interfaces between cells, and adherens junctions form laterally, characterized by E-cadherin localization (Fig. 1C). The endoderm layer is separated from the underlying mesoderm-derived cells by a specialized extracellular matrix, the basement membrane (Fig. 1B)
Figure 1. Rho GTPase functions in epithelial development. (A) Rhou is expressed in the foregut (fg) endoderm (en) of mouse embryos at embryonic day 9.5. Expression is downregulated in the ventral midline where the thyroid bud (t) emerges. (B) The foregut endoderm consists of polarized columnar epithelial cells with F-actin (red, visualized with phalloidin) concentrated beneath the apical surface, and a basement membrane containing fibronectin (green, visualized by immunofluorescence). (C) The foregut endoderm forms tight junctions apically (red, visualized by ZO-1 immunofluorescence) and adherens junctions (green, visualized by E-cadheren immunofluorescence) laterally. (D) Rho GTPases have multiple functions in epithelia. Rho GTPases interact with effector proteins to influence cell polarity, actin localization and cell junction formation. In turn, this affects tissue organization and lineage differentiation. (E and F) Electroporation of a construct encoding GFP-tagged Rhou (green) into the foregut endoderm reveals that its subcellular distribution overlaps with that of F-actin (red). (D) Merged image of GFP and phalloidin staining. (E) Phalloidin staining only. (B and C; E and F) Apical aspect is at the top of the figure, basal is at the bottom.
Rho GTPases play multiple roles in regulating the cytoskeletal organization and polarity of epithelial cells, which impact on tissue morphogenesis and cellular differentiation (Fig. 1D). In early Drosophila embryos, binding of Cdc42 to Par6 is essential for the apical localization of Par6 and aPKC in epithelial cells.1 Cdc42 plays a similar role in establishing the epithelial architecture of the epiblast layer in early post-implantation mouse embryos.2,3 In Xenopus embryos, Rho-related proteins are involved in the morphogenesis of the endoderm as this tissue transforms from a solid rod into an epithelial tube. Inhibiting Rho GTPase activity disrupts epithelialization and subsequently the elongation of the gut tube.4 The role of Cdc42 or other related Rho GTPases in the morphogenesis and differentiation of the endoderm in mammalian embryos has not been fully established. Our recent study5 has offered a glimpse of the potential role of a Cdc42-related protein, Rhou, in maintaining the epithelial structure and in differentiation of the foregut endoderm.
Rhou: An Atypical GTPase in the Endoderm
In a microarray analysis of the transcriptome of embryonic foregut endoderm, Rhou was identified among the genes that are expressed at a higher level in the foregut endoderm than other tissues. The expression of Rhou in the endoderm is initiated at a time when the foregut pocket is being formed from this tissue in a process involving extensive morphogenetic tissue movement. Within this region, cells in the ventral and lateral regions change in appearance from a flattened, squamous epithelium to a polarized columnar epithelium that displays apical polarization of the F-actin (Fig. 1A–C). In these cells, the distribution of GFP-tagged Rhou protein overlaps with that of F-actin and like F-actin, Rhou is enriched in the apical domain (Fig. 1E and F).
Rhou and its close relative Rhov are atypical Rho GTPases. Rhou has a higher GTP exchange rate than “classical” Rho GTPases, suggesting that it exists primarily in the active, GTP bound form. Rhou and Rhov have unique N-terminal sequences that regulate their activity and bind to adaptor proteins,6,7 and C-terminal motifs that are involved in protein localization.8 Previous work has shown that, when expressed in various types of cultured cells, Rhou can influence F-actin distribution, cell adhesion, cell motility and inter-cellular junction formation.9,10 Of particular relevance to tissue morphogenesis, knockdown of Rhou in MDCK cells, an epithelial cell model, impairs their ability to form epithelial cysts.10
Rhou Functions in Endoderm Differentiation
We investigated the function of Rhou in cell differentiation and embryonic development using embryonic stem (ES) cell lines in which Rhou activity was stably knocked down. The ability of these Rhou-knockdown ES cells to differentiate was examined by monitoring their differentiation in vitro as embryoid bodies (EBs). In parallel, embryos were directly derived from these cells by tetraploid complementation,11 allowing us to examine the consequences of reduced Rhou activity for development of the foregut and the embryo as a whole. Our results show that in the Rhou knockdown embryos, endoderm cells in the foregut lost their proper columnar epithelial organization and the gut acquired a deflated shape. While tight and adherens junctions appeared to form normally, the distribution of F-actin was no longer strongly apically polarized and the cells were depleted of microvilli on their apical surface. In embryos, the liver and thyroid buds were still able to form but were morphologically abnormal. Genes that are expressed specifically in the foregut endoderm or the liver bud (Pyy, Igfbp5, Pax9 and Apom) showed reduced expression. In the EBs, the expression of endoderm-derived hepatic (Hhex, Mug1, Ttr) and pancreatic (Iapp, Pdx1) lineage markers was reduced. Therefore, Rhou is required for regulating epithelial morphogenesis and endoderm differentiation.
Rhou has previously been shown to be capable of activating JNK,12 which could mediate its effects on the actin cytoskeleton and migration of cultured cells. The influence of Rhou on differentiation could therefore be, at least in part, mediated by its effects on JNK activity (Fig. 2A). In our in vitro differentiation experiments Ttr, Nrp1 and Wnt5a, which are all transcriptional targets of the AP-1 transcription factor complex or its constituent protein c-Jun, were expressed at a lower level in Rhou knockdown EBs. Activated of c-Jun requires JNK-dependent phosphorylation. Complete loss of JNK1 and JNK2 activity by genetic knockout of the Mapk8 and Mapk9 genes results in embryonic lethality with defective neural tube development, although the effects on endoderm differentiation and endodermal organ development have not been investigated.13,14
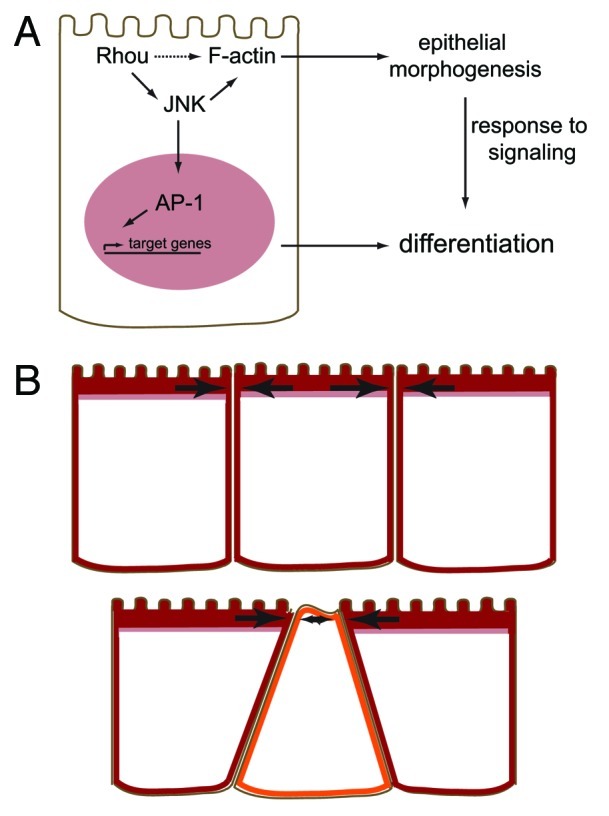
Figure 2. Rhou expression influences epithelial morphogenesis, differentiation and organ budding in the endoderm. (A) Rhou influences the localization of F-actin in the cell, possibly via interactions with unidentified effector proteins (dotted arrow) and/or by promoting JNK activity. JNK activates c-Jun, a part of the AP-1 transcription factor complex, which could directly affect the expression of endodermal genes, and also influences the actin cytoskeleton which may indirectly affect the cell’s response to signaling. (B) When Rhou is expressed in the endoderm, the apical shroud of F-actin that it colocalizes with (red) helps to stabilize the cell against compressive forces (arrows, top). When Rhou is downregulated, there is less F-actin apically (orange cell), reducing the cells resistance to compressive forces which could result in an apical constriction of the cell, forcing the cell away from the apical surface (bottom). This is a possible mechanism for the initiation of organ budding from the endoderm.
In our study, reduction of JNK activity during embryoid body differentiation by a small molecule inhibitor reduced the expression of the endoderm lineage markers Pyy and Mug1 compared with controls;5 and in a previous study15 in which both Mapk8 and Mapk9 were knocked out, expression of Sox17, a marker of the early definitive endoderm, was reduced in embryoid bodies. This raises the possibility that Rhou-dependent JNK activity is critical for endoderm lineage differentiation, possibly due to the downstream effects of loss of AP-1 transcriptional activity (Fig. 2A). Indeed, Ttr, which is expressed in the liver and is downregulated in Rhou knockdown embryoid bodies, is a direct transcriptional target of AP-1.16
Also possible is that the defects in F-actin localization, cell shape and tissue architecture in Rhou deficient embryos contribute to the reduced capacity for differentiation to endodermal lineages (Fig. 2A). These defects may be downstream of impaired JNK activation, or due to the loss of interactions with other effector proteins. Defects in cytoskeletal polarity caused by Rho-related GTPase deficiency have previously been shown to affect differentiation. In Ciona intestinalis, altered Cdc42 expression affects the formation of polarized, invasive cell protrusions and the induction of cardiac progenitor differentiation.17 Cdc42 also influences the choice of erythroid vs. myeloid differentiation of hematopoietic stem cells18 and tissue-specific knockout of Cdc42 activity interferes with pancreatic tubulogenesis and differentiation.19
WNT Regulation of Rhou and Endoderm Development
Rhou was originally identified in a screen for genes that are upregulated in response to WNT1.12 Consistent with this we found that embryos carrying mutations that increased β-catenin-dependent (canonical) WNT signaling activity resulted in upregulation of Rhou expression. We also observed upregulation of Rhou in cells cultured in the presence of Wnt3a, which is thought to act via the β-catenin-dependent pathway, and following transfection with a construct encoding constitutively active β-catenin. In contrast Wnt5a, which acts primarily via β-catenin-independent means, did not cause upregulation of Rhou. Although Rhou expression is influenced by the level of canonical WNT signaling, it is not entirely dependent on β-catenin-mediated signaling since knockdown of Ctnnb1, which encodes β-catenin, had no significant effect on Rhou expression. On the other hand overexpression of Sox17, which can interfere with canonical WNT signaling through its interaction with β-catenin,20 caused a reduction in Rhou expression.
β-catenin-dependent WNT signaling influences cell proliferation, fate and differentiation21 and in this context WNT signaling could play critical roles at multiple stages of foregut endoderm development. Deletion of Ctnnb1 early in post-implantation development results in a change of cell morphology of the endoderm from epithelial to mesenchymal and biases the cell fate toward heart mesoderm.22 Consistent with this, WNT signaling is required for the differentiation of ES cells into foregut endoderm.23 WNT signaling is later required for the development of the exocrine pancreas,24 lung25 and liver.26
Our work further suggests a link between the regulation of gene expression by canonical WNT signaling and the cellular effects more commonly associated with WNT-regulated planar cell polarity (PCP), which occurs via β-catenin-independent pathways. Expression of Rhou in the endoderm influences the actin cytoskeleton, cell shape and epithelial morphogenesis. This may occur through its effects on JNK activity, which could influence the expression of transcriptional targets of AP-1/c-Jun and also directly affect the cytoskeleton. Of particular interest is that one of the AP-1 targets that is downregulated in Rhou knockdown EBs is Wnt5a.5,27 Wnt5a functions in the PCP pathway in mice28 and can regulate the actin cytoskeleton in polarized cell types, including podocytes in mouse kidneys.29 Components of the WNT/PCP pathway regulate planar polarity (directional polarity within the plane of a layer of cells) and also apical-basal polarity,30 which suggests that changes in Wnt5a expression in Rhou knockdown cells could contribute to the abnormal apical-basal polarity of the actin cytoskeleton.
Implications for Organ Bud Formation
The thyroid bud develops from a patch of cells in the ventral midline of the foregut endoderm, forming a pseudostratified epithelium into which surrounding cells are recruited, to form a multi-layered epithelium that grows into an elongated bud which extends into the surrounding mesenchyme.31 The cells in the early thyroid bud continue to express the epithelial marker E-cadherin, but not N-cadherin which is expressed in surrounding mesenchymal cells.32 A similar process of pseudostratification preceding multilayering and outgrowth occurs during the formation of the liver, in a process that depends on the homeobox gene Hhex.33 In vitro experimental work suggests that the formation of a bud involves cell shape changes which could involve alterations to the cytoskeleton.34
During normal development, Rhou is downregulated in the ventral midline endoderm at the point where the thyroid primordium is beginning to form (Fig. 1A) and we observed that knocking down of Rhou in individual cells by electroporation of shRNA constructs into the foregut endoderm, or in chimeras between Rhou-knockdown ES cells and wild-type host embryos, resulted in a greater tendency for the Rhou-deficient cells to withdraw from the apical domain of the epithelium and be relocated toward the basal side of the epithelium. This may mirror the normal cellular behavior of endoderm cells in the foregut as they downregulate Rhou and engage in the formation of organ buds. It may be that downregulation of Rhou is a key step in the formation of organ buds in from the endoderm.
Apical constriction resulting from increases in cortical tension inside the cell and mediated by actin and myosin is an important mechanism in tissue folding and invagination.35 Our study suggests an additional mechanism of apical compression may be involved in the initiation of the epithelial multilayering that heralds the start of organ budding. Localized reductions in Rhou expression, either during normal development, or experimentally induced, causes changes in the actin cytoskeleton in those cells, including reducing the apical F-actin content. F-actin plays an important role in elasticity and resistance to compressive forces in cells,36 and so a reduction in apical F-actin will weaken the resistance of a cell to compressive forces from a surrounding cell, resulting in a compression of the apical domain and forcing the cell away from the luminal surface (Fig. 2B). The organ bud expands partly by recruitment of surrounding cells, so any cells surrounding the nascent primordium that downregulate Rhou will also become part of the multilayered structure. We propose that Rhou has a critical function in the regulation of the induction of budding from a single layer epithelium and could also be involved controlling the expansion of the bud.
Acknowledgments
Images in Figure 1A–C were taken by Josh Studdert. Our work was supported by grants from the National Health and Medical Research Council (grant 323704), the Australian Research Council (DP0985052) and Mr James Fairfax. D.A.F.L. is a Kimberly-Clark Research Fellow. P.P.L.T. is an NHMRC Senior Principal Research Fellow.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/18820
References
- 1.Hutterer A, Betschinger J, Petronczki M, Knoblich JA. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell. 2004;6:845–54. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Li S, Chrostek-Grashoff A, Czuchra A, Meyer H, Yurchenco PD, et al. Cdc42 is crucial for the establishment of epithelial polarity during early mammalian development. Dev Dyn. 2007;236:2767–78. doi: 10.1002/dvdy.21309. [DOI] [PubMed] [Google Scholar]
- 3.Chen F, Ma L, Parrini MC, Mao X, Lopez M, Wu C, et al. Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr Biol. 2000;10:758–65. doi: 10.1016/S0960-9822(00)00571-6. [DOI] [PubMed] [Google Scholar]
- 4.Reed RA, Womble MA, Dush MK, Tull RR, Bloom SK, Morckel AR, et al. Morphogenesis of the primitive gut tube is generated by Rho/ROCK/myosin II-mediated endoderm rearrangements. Dev Dyn. 2009;238:3111–25. doi: 10.1002/dvdy.22157. [DOI] [PubMed] [Google Scholar]
- 5.Loebel DA, Studdert JB, Power M, Radziewic T, Jones V, Coultas L, et al. Rhou maintains the epithelial architecture and facilitates differentiation of the foregut endoderm. Development. 2011;138:4511–22. doi: 10.1242/dev.063867. [DOI] [PubMed] [Google Scholar]
- 6.Shutes A, Berzat AC, Cox AD, Der CJ. Atypical mechanism of regulation of the Wrch-1 Rho family small GTPase. Curr Biol. 2004;14:2052–6. doi: 10.1016/j.cub.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JS, Koenig A, Young C, Billadeau DD. GRB2 couples RhoU to epidermal growth factor receptor signaling and cell migration. Mol Biol Cell. 2011;22:2119–30. doi: 10.1091/mbc.E10-12-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berzat AC, Buss JE, Chenette EJ, Weinbaum CA, Shutes A, Der CJ, et al. Transforming activity of the Rho family GTPase, Wrch-1, a Wnt-regulated Cdc42 homolog, is dependent on a novel carboxyl-terminal palmitoylation motif. J Biol Chem. 2005;280:33055–65. doi: 10.1074/jbc.M507362200. [DOI] [PubMed] [Google Scholar]
- 9.Aspenström P, Ruusala A, Pacholsky D. Taking Rho GTPases to the next level: the cellular functions of atypical Rho GTPases. Exp Cell Res. 2007;313:3673–9. doi: 10.1016/j.yexcr.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Brady DC, Alan JK, Madigan JP, Fanning AS, Cox AD. The transforming Rho family GTPase Wrch-1 disrupts epithelial cell tight junctions and epithelial morphogenesis. Mol Cell Biol. 2009;29:1035–49. doi: 10.1128/MCB.00336-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy A, Gocza E, Diaz EM, Prideaux VR, Ivanyi E, Markkula M, et al. Embryonic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–21. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- 12.Tao W, Pennica D, Xu L, Kalejta RF, Levine AJ. Wrch-1, a novel member of the Rho gene family that is regulated by Wnt-1. Genes Dev. 2001;15:1796–807. doi: 10.1101/gad.894301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabapathy K, Jochum W, Hochedlinger K, Chang L, Karin M, Wagner EF. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev. 1999;89:115–24. doi: 10.1016/S0925-4773(99)00213-0. [DOI] [PubMed] [Google Scholar]
- 14.Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–76. doi: 10.1016/S0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 15.Xu P, Davis RJ. c-JunNH2-terminal kinase is required for lineage-specific differentiation but not stem cell self-renewal. Mol Cell Biol. 2010;30:1329–40. doi: 10.1128/MCB.00795-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian X, Samadani U, Porcella A, Costa RH. Decreased expression of hepatocyte nuclear factor 3 alpha during the acute-phase response influences transthyretin gene transcription. Mol Cell Biol. 1995;15:1364–76. doi: 10.1128/mcb.15.3.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooley J, Whitaker S, Sweeney S, Fraser S, Davidson B. Cytoskeletal polarity mediates localized induction of the heart progenitor lineage. Nat Cell Biol. 2011;13:952–7. doi: 10.1038/ncb2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, Wang L, Kalfa TA, Cancelas JA, Shang X, Pushkaran S, et al. Cdc42 critically regulates the balance between myelopoiesis and erythropoiesis. Blood. 2007;110:3853–61. doi: 10.1182/blood-2007-03-079582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesavan G, Sand FW, Greiner TU, Johansson JK, Kobberup S, Wu X, et al. Cdc42-mediated tubulogenesis controls cell specification. Cell. 2009;139:791–801. doi: 10.1016/j.cell.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 20.Chew LJ, Shen W, Ming X, Senatorov VV, Jr., Chen HL, Cheng Y, et al. SRY-box containing gene 17 regulates the Wnt/β-catenin signaling pathway in oligodendrocyte progenitor cells. J Neurosci. 2011;31:13921–35. doi: 10.1523/JNEUROSCI.3343-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan CY, Nusse R. The WNT signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 22.Lickert H, Cox B, Wehrle C, Taketo MM, Kemler R, Rossant J. Dissecting Wnt/beta-catenin signaling during gastrulation using RNA interference in mouse embryos. Development. 2005;132:2599–609. doi: 10.1242/dev.01842. [DOI] [PubMed] [Google Scholar]
- 23.Hansson M, Olesen DR, Peterslund JM, Engberg N, Kahn M, Winzi M, et al. A late requirement for Wnt and FGF signaling during activin-induced formation of foregut endoderm from mouse embryonic stem cells. Dev Biol. 2009;330:286–304. doi: 10.1016/j.ydbio.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells JM, Esni F, Boivin GP, Aronow BJ, Stuart W, Combs C, et al. Wnt/beta-catenin signaling is required for development of the exocrine pancreas. BMC Dev Biol. 2007;7:4. doi: 10.1186/1471-213X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–8. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bi Y, Huang J, He Y, Zhu GH, Su Y, He BC, et al. Wnt antagonist SFRP3 inhibits the differentiation of mouse hepatic progenitor cells. J Cell Biochem. 2009;108:295–303. doi: 10.1002/jcb.22254. [DOI] [PubMed] [Google Scholar]
- 27.Gerdes MJ, Myakishev M, Frost NA, Rishi V, Moitra J, Acharya A, et al. Park Sw, Glick A, Yuspa SH, Vinson C. Activator Protein-1 Activity Regulates Epithelial Tumor Cell Identity. Cancer Res. 2006;66:7578–88. doi: 10.1158/0008-5472.CAN-06-1247. [DOI] [PubMed] [Google Scholar]
- 28.Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–33. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babayeva S, Zilber Y, Torban E. Planar cell polarity pathway regulates actin rearrangement, cell shape, motility, and nephrin distribution in podocytes. Am J Physiol Renal Physiol. 2011;300:F549–60. doi: 10.1152/ajprenal.00566.2009. [DOI] [PubMed] [Google Scholar]
- 30.Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn. 2005;232:685–94. doi: 10.1002/dvdy.20334. [DOI] [PubMed] [Google Scholar]
- 31.Fagman H, Nilsson M. Morphogenesis of the thyroid gland. Mol Cell Endocrinol. 2010;323:35–54. doi: 10.1016/j.mce.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Fagman H, Grande M, Edsbagge J, Semb H, Nilsson M. Expression of classical cadherins in thyroid development: maintenance of an epithelial phenotype throughout organogenesis. Endocrinology. 2003;144:3618–24. doi: 10.1210/en.2003-0393. [DOI] [PubMed] [Google Scholar]
- 33.Bort R, Signore M, Tremblay K, Martinez Barbera JP, Zaret KS. Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev Biol. 2006;290:44–56. doi: 10.1016/j.ydbio.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Hilfer SR, Palmatier BY, Fithian EM. Precocious evagination of the embryonic chick thyroid in ATP-containing medium. J Embryol Exp Morphol. 1977;42:163–75. [Google Scholar]
- 35.Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–44. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- 36.Ofek G, Wiltz DC, Athanasiou KA. Contribution of the cytoskeleton to the compressive properties and recovery behavior of single cells. Biophys J. 2009;97:1873–82. doi: 10.1016/j.bpj.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]