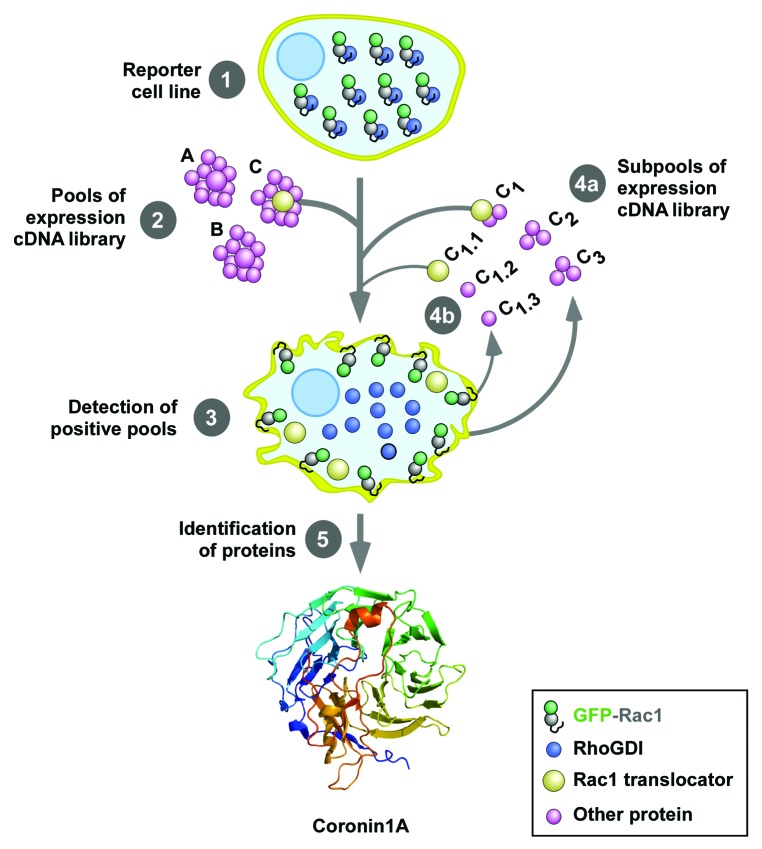
Figure 2. Scheme of the cellomic screen used in the work reviewed here. The first screening was conducted using an expression library of 135,000 independent cDNA clones obtained from human T cells. To this end, we used a reporter HEK293T stably expressing a cytoplasmic EGFP-Rac1 protein (step 1). To increase the efficiency of the screening, this cell line also expressed anti-apoptotic proteins to avoid the loss of clones due to the presence of pro-apoptotic molecules in the transfected cDNA pools. The screening was conducted by transfecting separate pools of 90 cDNAs in the reporter cell line using the calcium phosphate precipitation method (step 2) and the subsequent score of cells showing plasma membrane localized EGFP-Rac1 using epifluorescence microscopy (step 3). Positive pools were progressively subdivided and transfected in the reporter cell line (steps 4a and 4b) until the isolation of the cDNA clones responsible for the Rac1 translocating activity (step 5). The crystal structure of Coro1A, one of the clones identified in this screening, is shown at the bottom. The structure is composed of WD40 domains arranged in a prototypical β-propeller conformation. See inset at the bottom for the shape and color code use for the indicated proteins used in these experiments.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.