Abstract
Tibouchina granulosa (Melastomataceae), Brazilian glorytree (Brazilian common name – quaresmeira), a common tree of the Atlantic Forest of Brazil, is widely used as an ornamental for its violet or pink blossoms. Little is known about fungal diseases affecting this species, although these represent a known limitation for its cultivation in nurseries. Among these there is a foliage blight that occurs in combination with distortion of branch apices and die-back. A consistent association of a species of Pilidiella with the diseased tissues was observed. The fungus was isolated in pure culture and based on its morphology and DNA phylogeny, we conclude that it represents a new species, for which the name Pilidiella tibouchinae is introduced.
Keywords: Coniella, Diaporthales, ITS, LSU, Sordariomycetes, systematics
INTRODUCTION
Tibouchina granulosa (Melastomataceae), the Brazilian glorytree, is a fast growing tree native that occurs in the Atlantic Forest of Brazil. It is a common and important component of the native flora, particularly in secondary forests. It is also a highly prized ornamental, which is widely used in gardens and parks because of its spectacular violet or pink blossoms that each year appear prior and during Easter. The period of lent (quaresma, in Portuguese) is an important period in the catholic tradition, from which the Brazilian common name for this tree, “quaresmeira” is derived (Lorenzi 2002). Usually there is a high demand for young T. granulosa plants in the garden nursery market in Brazil, particularly for the variety that produces pink flowers. Nevertheless, diseases are known to be a limiting factor in nursery production of T. granulosa. However, very little is known about the diseases affecting this tree. The record of Chrysoporthe cubensis (syn.: Cryphonectria cubensis) on T. granulosa in Brazil by Seixas et al. (2004) is the sole record of a fungal pathogen on this host in the Brazilian database (http://pragawall.cenargen.embrapa.br/aiqweb/michtml/fichahp.asp?id=1912)–and there are only three records of fungi on this host in the USDA fungal database (Farr & Rossman 2010). This is somewhat surprising for such a common plant in the neotropics, and possibly reflects the limited existing knowledge about plant pathogenic fungi occurring on wild plants in this region. Here we clarify the identity of the fungus associated with a severe foliage blight (often evolving into a form of die-back) of quaresmeira. This is one of the most widespread and damaging diseases affecting T. granulosa in the field, in gardens, and also in nurseries.
MATERIAL AND METHODS
Isolates
Samples of young abnormal branches of Tibouchina granulosa bearing diseased leaves were collected at two localities in Brazil (states of Rio de Janeiro and Minas Gerais), dried in a plant press and brought to the laboratory for further examination. Representative specimens of the fungus were deposited in the herbarium at the Universidade Federal de Viçosa (VIC). Pure cultures were obtained by transfer of conidia, using a sterile fine-pointed needle, from lesions onto plates containing VBA (vegetable broth-agar) as described in Pereira et al. (2003). Pure cultures are deposited in the fungal culture collection at the Universidade Federal de Viçosa and also at the CBS-KNAW Fungal Biodiversity Centre (CBS) in Utrecht, The Netherlands. Representative voucher specimens are deposited in VIC and at CBS.
DNA isolation, amplification and analyses
Genomic DNA was isolated from fungal mycelium grown on MEA, using the UltraCleanTM Microbial DNA Isolation Kit (MoBio Laboratories, Inc., Solana Beach, CA, USA) according to the manufacturer’s protocols. The primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990) were used to amplify part of the nuclear rDNA operon spanning the 3′ end of the 18S rRNA gene (SSU), the internal transcribed spacer 1, the 5.8S rRNA gene, the internal transcribed spacer 2 (ITS) and the first 900 bases at the 5′ end of the 28S rRNA gene (LSU). The primers ITS4 (White et al. 1990) and LSU1Fd (Crous et al. 2009a) were used as internal sequence primers to ensure good quality sequences over the entire length of the amplicon. The PCR conditions, sequence alignment, and subsequent phylogenetic analysis followed the methods of Crous et al. (2006, 2009b). Additionally, partial translation elongation factor 1-alpha (TEF) sequences were determined as described by Bensch et al. (2010). Sequences were compared with the sequences available in NCBI’s GenBank nucleotide (nr) database using a megablast search and alignments were constructed based on these results for ITS. For LSU, the novel sequence were added to an alignment modified from Lamprecht et al. 2011 (TreeBASE study S11805). Sequences derived in this study were lodged at GenBank, the alignment in TreeBASE (www.treebase.org/treebase/index.html), and taxonomic novelties in MycoBank (www.MycoBank.org; Crous et al. 2004).
Morphology
Slides containing fungal structures were mounted in lactophenol or lactofuchsin, with 30 measurements determined per structure. Sections were prepared with the help of a freezing microtome (Microm HM 520). Observations of fungal structures and measurements, as well as preparation of photographs, were performed with an Olympus BX 51 light microscope fitted with an Olympus E330 camera. Colony characters and pigment production were noted after 6 d of growth on 2 % malt extract agar (MEA) and potato carrot agar (PCA) (Crous et al. 2009c) plates incubated at 25 °C. Colony colours (surface and reverse) were rated according to the colour charts of Rayner (1970).
RESULTS
Phylogeny
Approximately 1700 bases, spanning the ITS and LSU regions, were obtained from the sequenced culture. The ITS region was used in the phylogenetic analysis to determine species-rank relationships (Fig. 1) and the LSU region for the generic placement (Fig. 2). The manually adjusted ITS alignment contained 25 taxa including the outgroup sequence and, of the 501 characters used in the phylogenetic analysis, 86 were parsimony-informative, 49 were variable and parsimony-uninformative, and 366 were constant. Twenty-four equally most parsimonious trees were retained from the heuristic search, the first of which is shown in Fig. 1. The phylogenetic tree of the ITS region (Fig. 1) shows that the obtained sequences cluster between Pilidiella crousii and Pilidiella granati. The manually adjusted LSU alignment contained 54 taxa including the two outgroup sequences and, of the 840 characters used in the phylogenetic analysis, 190 were parsimony-informative, 54 were variable and parsimony-uninformative, and 596 were constant. From this heuristic search, 180 equally most parsimonious trees were retained, the first of which is shown in Fig. 2. Phylogenetic analysis of the LSU region (Fig. 1) confirms the placement of the novel sequences in Pilidiella. The partial TEF sequences did not have any high identity to those sequences available in GenBank (data not shown).
Fig. 1.
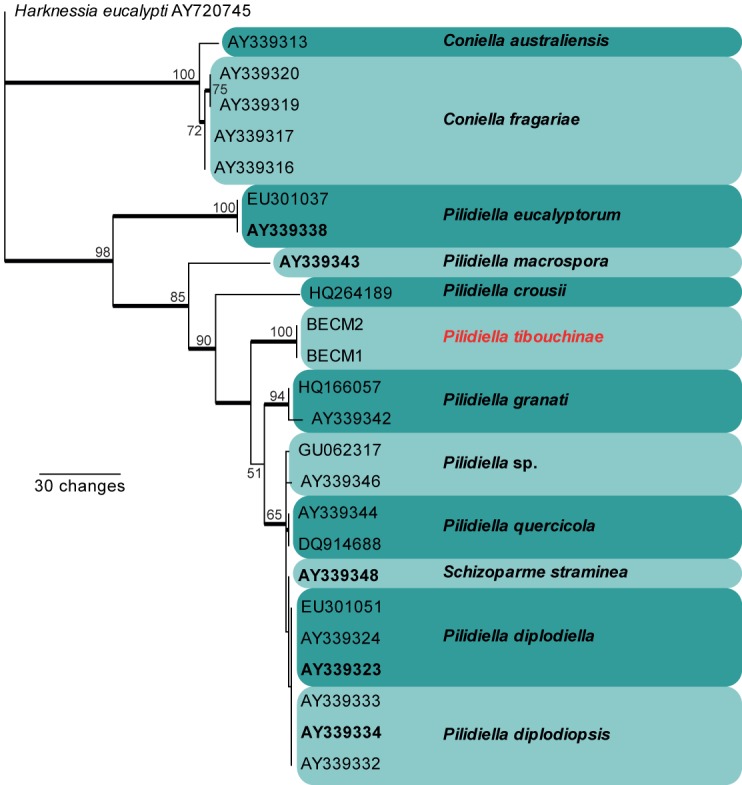
The first of 24 equally most parsimonious trees obtained from a heuristic search with 50 random taxon additions of the ITS sequence alignment (Tree length = 229, CI = 0.834, RI = 0.904, RC = 0.754). The scale bar shows 30 changes, and bootstrap support values from 1000 replicates are shown at the nodes. Accession numbers of ex-type strains are shown in bold and the novel species in this study in red. Branches present in the strict consensus tree are thickened and the tree was rooted to a sequence of Harknessia eucalypti (GenBank accession no. AY720745).
Fig. 2.
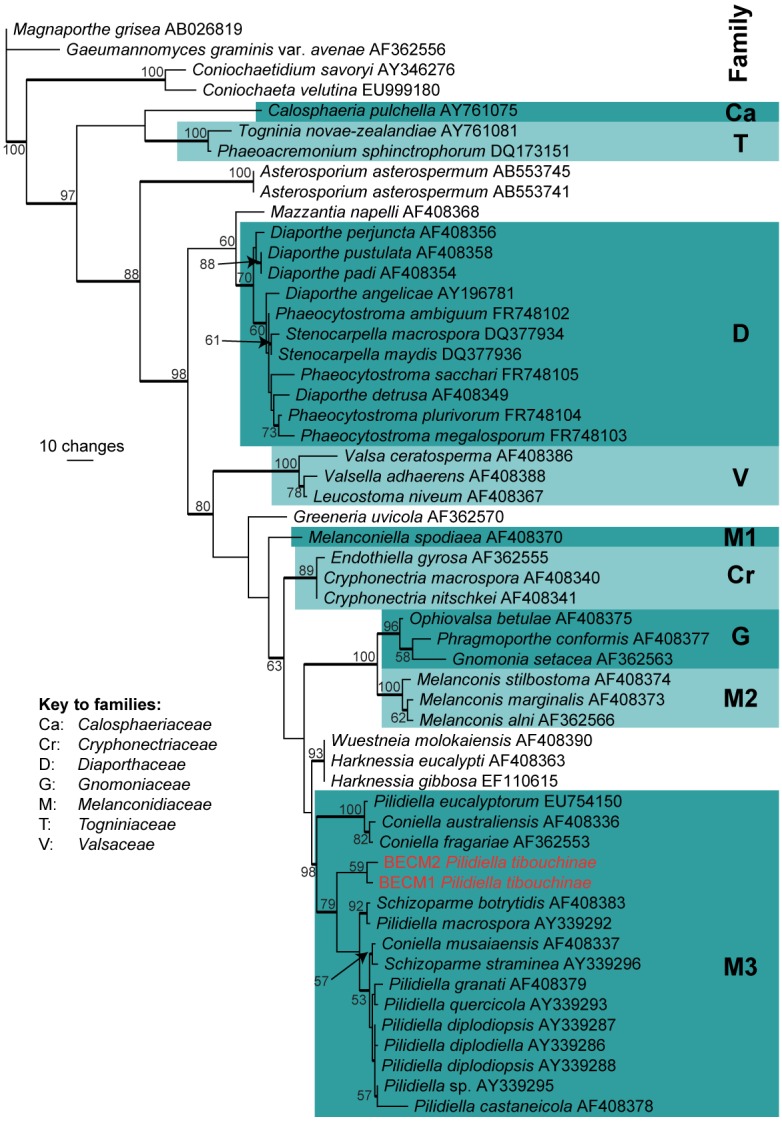
The first of 180 equally most parsimonious trees obtained from a heuristic search with 100 random taxon additions of the LSU sequence alignment (Tree length = 594, CI = 0.515, RI = 0.817, RC = 0.421). The scale bar shows 10 changes, and bootstrap support values from 1000 replicates are shown at the nodes. The novel species in this study is indicated in red and families are indicated to the right of the tree according to the key on the figure. Branches present in the strict consensus tree are thickened and the tree was rooted to sequences of Magnaporthe grisea and Gaeumannomyces graminis var. avenae (GenBank accession nos AB026819 and AF362556, respectively).
Taxonomy
A pycnidial coelomycete was regularly associated with diseased tissues on the samples collected at the two separate localities. Its morphology conformed to that of species in the genus Pilidiella (Nag Raj 1993, van Niekerk et al. 2004), although it appeared to represent a distinct taxon. Accordingly, a new species name is introduced below to accommodate the fungus occurring on T. granulosa.
Pilidiella tibouchinae B.E.C. Miranda, R.W. Barreto & Crous, sp. nov.
MycoBank MB563992
(Fig. 3)
Fig. 3.
Pilidiella tibouchinae. A–D. Leaf spots and curling on Tibouchina granulosa. E. Colony on oatmeal agar. F, G. Vertical section through pycnidia. H, I. Conidiogenous cells. J, K. Conidia. Bars = 10 μm.
Etymology: Named after the host genus on which it occurs, Tibouchina.
Diagnosis: Similar to Pilidiella eucalyptorum but lacking conidial germ slits, and similar to P. petrakioidea but lacking mucoid appendages on conidia.
Type: Brazil: Minas Gerais: Viçosa, campus of the Universidade Federal de Viçosa, on leaves of Tibouchina granulosa, 8 March 2010, B. C. Miranda (VIC 31443 – holotype; CBS H-20827 – isotype; cultures ex-holotype CPC 18511, CPC 18512 = CBS 131595).
(GenBank accession numbers for VIC 31443 and VIC 31444: ITS = JQ281774, JQ281775; LSU = JQ281776, JQ281777; TEF = JQ281778, JQ281779)
Other specimen examined: Brazil: Minas Gerais: Viçosa, campus of the Universidade Federal de Viçosa, on leaves of T. granulosa, 17 May 2010, B. C. Miranda (VIC 31444).
Description: Lesions on living leaves and young stems, firstly as adaxial straw-coloured necrotic spots, mostly appearing near the leaf veins, becoming yellowish to greyish with a dark brown to dark purple border, irregularly shaped, coalescing and leading to necrosis and distortion of large parts of the leaf lamina; loss of necrotic leaf parts usually creating the impression of insect damage. In conjunction to these symptoms, a shortening of branch internodes, leaf distortion, bud death, necrosis and die-back of young stems are also observed. Stunting and decline of severely affected plants are observed even for adult plants. Conidiomata pycnidial, adaxial, subcuticular, solitary, globose to depressed globose, 42.5–75 × 75–112.5 μm, wall composed of dark greyish brown textura angularis of 1–3 cell layers, 7–12 μm thick, dark brown; dehiscence ostiolate, central; conidiophores formed on a dense, basal, cushion-like aggregation of hyaline cells, mostly reduced to conidiogenous cells, subcylindrical, branched below, 8–15 × 3–4 μm, smooth, hyaline, 1–2-septate. Conidiogenous cells enteroblastic, phialidic with apical periclinal thickening, 5–10 × 2–3 μm, smooth, hyaline, with minute collarette, and covered in mucilage. Conidia mostly broadly ellipsoidal, often somewhat flattened on one side, oblong, subreniform, ovoid to subovoid, 10–13 × 6–8 μm (l:b = 1.7), apex rounded, subtruncate at base, hilum sometimes slightly protuberant, aseptate, hyaline when immature, becoming smoky-brown at maturity, smooth, guttulate (usually with one large guttule but sometimes biguttulate or eguttulate).
Culture characteristics: (MEA or PCA either under a 12 h light regime or in the dark): Colonies fast-growing (up to 86 mm diam after 6 d); flat, occasionally slightly raised centrally; mostly composed of immersed mycelium, aerial mycelium mostly sparse (but very dense cottony to woolly aerial mycelium on MEA in the dark); cottony to woolly to spider web-like white to grey olivaceous, sometimes with some small cinnamon areas centrally, occasionally becoming powdery towards the periphery, abundant olivaceous black fruit bodies crowded in zone rings on MEA/light. On PCA greyish black to greenish black centrally, with saffron margin in reverse; black fruit bodies less abundant, and in more distinct rings on PCA/light.
DISCUSSION
The fungus on Tibouchina granulosa clearly belongs to the Coniella/Pilidiella-complex that has Schizoparme teleomorphs (Schizoparmaceae, Diaporthales; Rossman et al. 2007). Fungi in Schizoparmaceae include several species associated with foliar diseases, sometimes occurring as secondary invaders of plant tissues infected by other organisms or injured by other causes (Ferreira et al. 1997). There is no record of any teleomorphic species of Schizoparmaceae in association with members of the genus Tibouchina, and a single doubtful record of a Coniella on another member of the Melastomataceae, Miconia serrulata (Farr & Rossman 2010). Several members of Myrtales, which according to Bremer et al. (2003) includes up to 14 families, are known hosts of Schizoparmaceae (Farr & Rossman 2010). For instance, several species are known from Myrtaceae (Acca, Blepharocalyx, Eucalyptus, Eugenia, Heteropyxis, Myrcia, Syzygium), Lythraceae (Lythrum, Punica), and Combretaceae (Anogeissus, Anogeissus, Terminalia) (van Niekerk et al. 2004, Farr & Rossman 2010)
Sutton (1980) and Nag Raj (1993) treated Pilidiella as a synonym of Coniella. However, based on analyses of large subunit (LSU) nuclear ribosomal DNA (nrDNA) sequences, Castlebury et al. (2002) concluded that Pilidiella is distinct from Coniella. Pilidiella has two main morphological criteria separating it from Coniella: the presence of conidia that are hyaline when young becoming pale brown with age (consistently brown in Coniella) (Castlebury et al. 2002) and having a length to breadth ratio larger than 1.5 (equal to or smaller than 1.5 for Coniella) (van Niekerk et al. 2004). Pigmentation alone is difficult to interpret (Table 1), although P. tibouchinae has hyaline conidia that become smoky brown at maturity and a l:b ratio of 1.7. Additionally the results of the phylogenetic analysis place P. tibouchinae in the Pilidiella clade, distinct from Coniella (Figs 1–2). Considering the combination of morphological and molecular data, we prefer to place this fungus in the genus Pilidiella. Nevertheless, several species in the group still need to be re-examined, as is evident from Fig. 2, where Pilidiella eucalyptorum clusters in the Coniella clade, and C. musariensis clusters in the Pilidiella clade.
Table 1. Conidial morphology of selected Coniella and Pilidiella species recorded from members of Myrtales.
| Species | Size | l:b rate | Shape | Appendage | Germ slit | Reference |
|---|---|---|---|---|---|---|
| Coniella australiensis | (9−)10–11(−14) × (6−)7–8(−10) μm | 1.4 | Broadly ellipsoidal | + | - | van Niekerk et al. (2004) |
| C. castanaeicola | 13–29 × 2.5–3.5 μm | 7.3 | Fusoid to falcate | + | - | Nag Raj (1993) |
| C. costae | 19–28 × 7–7.5 μm | 3.2 | Fusoid to ellipsoid | - | - | Dianese et al. (1993) |
| C. delicata | 7–9 × 2.5–3 μm | 2.9 | Ellipsoid | - | - | Sutton (1980) |
| C. fragariae | (8−)9–10(−12.5) × (5−)6–7(−8) μm | 1.5 | Ellipsoid | + | + | van Niekerk et al. (2004) |
| in older conidia | ||||||
| C. macrospora | (18.3−)25–29(−32.5) × (13−)16–20(−21.5) μm | 1.5 | Ovoid, ellipsoid, pyriform, globoid | + | - | van der Aa (1983) |
| C. minima | 6.5–7.5 × 3.5–4.5 μm | 1.5 | Globoid to subgloboid | - | - | Sutton (1969) |
| C. terminaliae | 2–8 × 2–3.5 μm | 2:01 | Globose to subglobose | - | - | Firdousi et al. (1994) |
| Pilidiella crousii | (6−)7–12(−13.5) × (2.5−)3–5 μm | 2.2 | Narrowly ellipsoid to ellipsoid | - | - | Rajeshkumar et al. (2011) |
| P. diplodiella | (10−)12–15(−19) × (4−)5–6 μm | 2.3 | Narrowly ellipsoid | + | - | van Niekerk et al. (2004) |
| P.eucalyptorum | (9−)10–12(−14) × (6−)7–8 μm | 1.6 | Broadly ellipsoid or limoniform | uncommon | + | van Niekerk et al. (2004) |
| P. granati | 9–16 × 3–4.5 μm | 2.8 | Ellipsoid | + | - | Nag Raj (1993) |
| P. jambolana | 19–22 × 3.5–4 μm | 5.7 | Elongate-fusoid | - | - | Ahmad (1967) |
| P. petrakioidea | 12–14.5 × 6.5–8 μm | 1.9 | Narrowly ellipsoid | + | - | Nag Raj (1993) |
| P. tibouchinae | 10–13 × 6–8 μm | 1.7 | Broadly ellipsoid | - | - | This publication |
Pycnidia in P. tibouchinae are small when compared to the species of Schizoparmaceae treated by Sutton (1980), Nag Raj (1993), and van Niekerk et al. (2004). Morphologically, conidia of P. tibouchinae show some similarity to that of P. eucalyptorum and P. petrakioidea. However, conidia of P. tibouchinae lack conidial germ slits (present in P. eucalyptorum) and mucoid appendages (present in P. petrakioidea). It also has thinner pycnidial walls (7–12 μm), than those in P. eucalyptorum (to 25 μm thick), and has hyaline to pale smoky-brown conidia, whereas those of P. eucalyptorum are medium to dark reddish brown. Furthermore, P. tibouchinae also differs from P. petrakioidea in conidial morphology (narrowly ellipsoidal with acutely rounded apices in P. petrakioidea) and a l:b ratio larger than 1.9. Five species of Coniella have been described in association with members of the Myrtaceae: C. australiensis, C. castaneicola, C. costae, C. fragariae, and C. minima. Considering the close morphological similarity of Pilidiella and Coniella, the conidial morphology of these species is also provided here for comparison with that of P. tibouchinae (Table 1).
Pilidiella tibouchinae is the first species of the genus to be described on a host belonging to Melastomataceae, on which it appears to be associated with a rather serious foliar and dieback disease. Further investigations aimed at clarifying the pathological status of the fungus on Tibouchina granulosa, and evaluating potential disease control measures are now urgently required, and will be reported elsewhere.
Acknowledgments
We thank the technical staff of CBS, Arien van Iperen (cultures), Marjan Vermaas (photographic plates), and Mieke Starink-Willemse (DNA isolation, amplification and sequencing) for their invaluable assistance.
REFERENCES
- Aa HA van der. (1983) A new species of Coniella. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen, C 86: 121–125 [Google Scholar]
- Ahmad S. (1967) Contributions to the fungi of West Pakistan–VI. Biologica 13: 15–42 [Google Scholar]
- Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, Summerell BA, Shin H-D, Dugan FM, Schroers H-J, Braun U, Crous PW. (2010) Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Studies in Mycology 67: 1–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer B, Bremer K, Chase MW, Reveal JL, Soltis DE, et al (2003) An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society 141: 399–436 [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. (2004) MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hyde KD. (2006) Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Studies in Mycology 55: 213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Summerell BA, Wingfield BD, Wingfield MJ. (2009b) Co-occurring species of Teratosphaeria on Eucalyptus. Persoonia 22: 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, Hoog GS De, Groenewald JZ. (2009a) Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (eds) (2009c) Fungal Biodiversity. [CBS Laboratory Manual Series 1.] Centraalbureau voor Schimmelcultures, Utrecht: [Google Scholar]
- Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN. (2002) A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031 [PubMed] [Google Scholar]
- Dianese JC, Medeiros RB, Santos LTP, Sutton BC. (1993) Coniella costae sp. nov. on leaves of Myrcia tomentosa from Brazilian cerrado. Mycological Research 97: 1234–1236 [Google Scholar]
- FAO (1996) International Standard for Phytosanitary Measures. Rome: Secretariat of the International Plant Protection Convention; [Google Scholar]
- Farr DF, Rossman AY. (2010) Fungal databases. Beltsville, MD: Systematic Mycology and Microbiology Laboratory, ARS, USDA; http://nt.ars-grin.gov/fungaldatabasews/ [Google Scholar]
- Ferreira FA, Alfenas AC, Coelho L. (1997) Portas-de-entrada para Coniella fragariae em folhas de eucalipto. Revista Árvore 21: 307–311 [Google Scholar]
- Firdousi SA, Sharma CD, Vyas KM. (1994) A new species of Coniella from India. Acta Botanica Indica 22: 134–135 [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. (1998) Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183–189 [DOI] [PubMed] [Google Scholar]
- Lamprecht SC, Crous PW, Groenewald JZ, Tewoldemedhin YT, Marasasm WFO. (2011) Diaporthaceae associated with root and crown rot of maize. IMA Fungus 2: 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi H. (2002) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas do Brasil. Vol. 1. 4a edição. Nova Odessa, SP: Instituto Plantarum; [Google Scholar]
- Nag Raj TR. (1993) Coelomycetous Anamorphs with Appendage-bearing Conidia. Waterloo: Mycologue Publications; [Google Scholar]
- Niekerk JM van, Groenewald JZ, Verkley GJM, Fourie PH, Wingfield MJ, Crous PW. (2004) Systematic reappraisal of Coniella and Pilidiella, with specific reference to species occurring on Eucalyptus and Vitis in South Africa. Mycological Research 108: 283–303 [DOI] [PubMed] [Google Scholar]
- Rayner RW. (1970) A Mycological Colour Chart. Kew: Commonwealth Mycological Institute; [Google Scholar]
- Rossman AY, Farr DF, Castlebury LA. (2007) A review of the phylogeny and biology of the Diaporthales. Mycoscience 48: 135–144 [Google Scholar]
- Sutton BC. (1969) Type studies of Coniella, Anthasthoopa, and Cyclodomella. Canadian Journal of Botany 47: 603–608 [Google Scholar]
- Sutton BC. (1980) The Coelomycetes: fungi imperfecti with pycnidia, acervuli and stromata. Kew: Commonwealth Mycological Institute; [Google Scholar]
- USDA/Fungal Database. Available at http://nt.ars-grin.gov/fungaldatabases/index.cfm (accessed 20 March 2010). [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee J, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: a guide to methods and applications: 315–322 San Diego: Academic Press; [Google Scholar]



