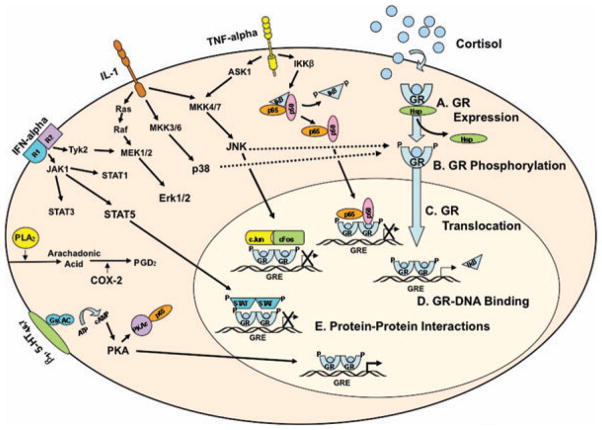
Figure 2.
Interactions between cytokine and glucocorticoid receptor (GR) signaling pathways. Selected cytokines and their signal transduction pathways are depicted in a simplified fashion to illustrate representative interactions between cytokine and GR signaling events. Cortisol binds to GR, resulting in dissociation of heat shock protein (HSP) complexes and subsequent phosphorylation. GR then translocates to the nucleus, where it dimerizes and either interacts with other transcription factors or binds to glucocorticoid response elements (GREs) upstream of GR-regulated genes (e.g., inhibitor κ-B or IκB). TNF-α binds to its receptor and results in activation of IκB kinase β (IKKβ), which phosphorylates IκB, allowing NF-κB (shown here as p65 and p50 Rel subunits) to translocate to the nucleus. Through protein-protein interactions, activated NF-κB associates with GR, thus interfering with GR-DNA binding. IL-1 binds to its receptor, initiating (a) mitogen activated protein kinase (MAPK) kinase (MKK)4/7, which culminates in activation of c-Jun amino-terminal kinase (JNK), (b) MKK3/6, which culminates in activation of p38, and (c) Ras, which results in activation of the extracellular signal-related kinase (Erk)1/2. Of note, MKK4/7 activation of JNK can also occur through TNF-α receptor binding. As depicted by the dotted lines, both p38 and JNK can phosphorylate key GR residues, thereby disrupting nuclear translocation of GR. Interferon (IFN)-α binds to its receptor resulting in Janus kinase (Jak) phosphorylation, represented as Jak1 and tyrosine kinase (Tyk)2. Jak1 phosphorylates signal transducers and activators of transcription (STAT) proteins, including STAT1, STAT3, and STAT5. Tyk2 can also activate elements of the Ras signaling pathway, resulting in activation of Erk1/2. Activated STATs translocate to the nucleus, where they can interact with GR through protein-protein interactions, thereby interfering with GR-DNA binding. Phospholipids are hydrolyzed by phospholipase A2 (PLA2) to form arachidonic acid, which is metabolized by cyclooxygenase (COX) 2 to produce prostaglandin D2 (PGD2). Stimulation of serotonergic receptors 4, 6, or 7 (5-HT4, 6, 7) and beta adrenergic receptors (beta1) induces a conformational change in G stimulatory (Gs) protein, which then activates adenylyl cyclase (AC). AC, in turn, converts adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). cAMP then induces a conformational change in protein kinase A (PKA), which translocates to the nucleus, where it is able to enhance GR-DNA binding. In addition, the catalytic subunit of PKA (PKAc) interacts with p65, thereby inhibiting NF-κB nuclear translocation. (Reprinted with permission from Pace, T. W. W., Hu, F., & Miller, A. H. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain, Behavior, and Immunity 21, 9–19, Copyright 2007 Elsevier.11) (In color in Annals online.)