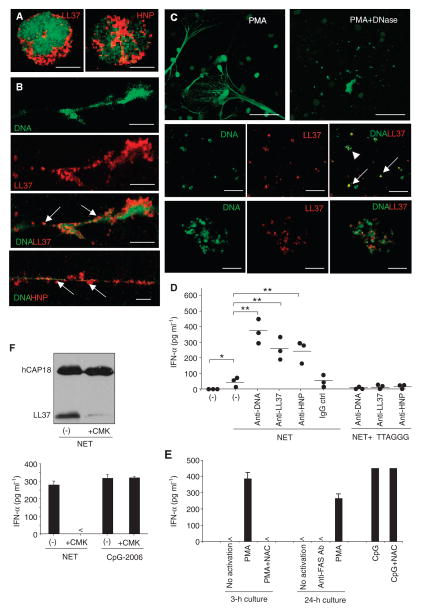
Fig. 5.
NETting neutrophils release self-DNA–antimicrobial peptide complexes that activate pDCs. (A to C) Confocal microscopy of unstimulated neutrophils (A), NETting neutrophils activated for 3 hours with phorbol 12-myristate 13-acetate (PMA) (B), or NETting neutrophils activated with PMA followed by treatment with DNase I (C), stained for DNA (green), LL37 (red), or HNPs (red) as indicated. Representative images are shown. Bars, 5 μm (A), 10 μm (B), 50 μm (C, upper panel), 20 μm (C, middle panel), and 4 μm (C, lower panel). Arrows indicate DNA–antimicrobial peptide complexes contained in NETs before (B) and after (C) DNase treatment. A high-power image of a complex indicated by the arrowhead is provided in the lower panel of (C). (D) IFN-α produced by pDCs stimulated with supernatants of NETting neutrophils alone or in the presence of anti-LL37, anti-HNP, anti-DNA, or control antibodies. In some experiments, the pDCs were pretreated with the TLR9 inhibitor ODN-TTAGGG. Each symbol represents an independent experiment, and horizontal bars represent the mean. *P = 0.023; **P < 0.04, ANOVA (Bonferroni adjustment). (E) Supernatants of neutrophils activated for 3 hours with PMA after pretreatment with N-acetyl-L-cysteine (NAC; 5 mM) to block NET formation, and supernatants of neutrophils stimulated for 24 hours with an anti-FAS antibody (Ab) to induce apoptosis, were used to stimulate pDCs. (F) Supernatants of NETting neutrophils pretreated with the serine protease inhibitor chloromethyl ketone (CMK) were used for immunoblot detection of LL37 and hCAP18 (upper panel) and to stimulate pDC for IFN-α production (lower panel). TLR9 agonist CpG-2006 was used as a positive control to exclude NET-independent effects of CMK on pDC activation. (E to F) One representative of at least three independent experiments is shown. Error bars represent the SD of triplicate wells.