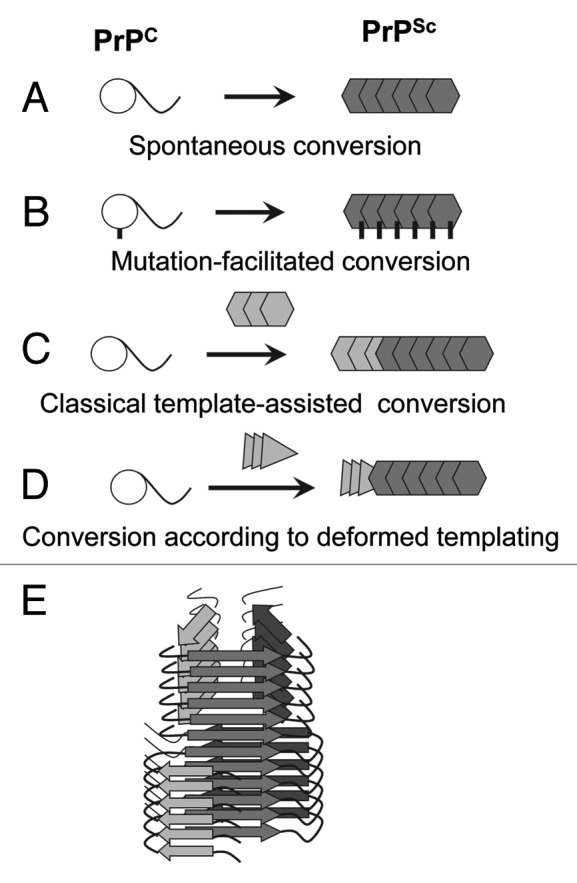
Figure 1. Four mechanisms for PrPSc formation. (A) Spontaneous conversion of PrPC into PrPSc is believed to underlie the sporadic forms of the prion diseases. (B) Disease-related mutations in the prion protein can facilitate the conversion of PrPC into PrPSc. (C) In prion diseases acquired via transmission, PrPSc replicates its pathogenic structure by recruiting and converting PrPC. According to the template-assisted model, the folding pattern of a newly recruited polypeptide chain accurately reproduces that of a PrPSc template. (D) A new mechanism referred to as “deformed templating” postulates that the formation of PrPSc de novo can be triggered by abnormal PrP structures substantially different from that of authentic PrPSc. Transformation from one cross-β folding pattern present in a template to a significantly different folding pattern, the one specific for PrPSc, occurs during deformed templating. (E) Schematic representation of a conformational switch in cross-β folding pattern within an individual fibril.
