Abstract
Good science and good animal care go hand in hand. A sick or distressed animal does not produce the reliable results that a healthy and unstressed animal produces. This unit describes the essentials of assessing mouse health, colony health surveillance, common conditions, and determination of appropriate endpoints. Understanding the health and well-being of the mice used in research enables the investigator to optimize research results and animal care.
Keywords: Mouse health evaluation
INTRODUCTION
Both investigative and veterinary staffs monitor the health and well-being of mice that are used in research. Indeed, this level of responsibility and care is mandated by the Public Health Service based on the Guide for the Care and Use of Laboratory Animals (National Research Council. 2011). The Guide is “intended to assist investigators in fulfilling their obligation to plan and conduct animal experiments in accord with the highest scientific, humane, and ethical principles.” All investigators should become familiar with the Guide (http://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf).
Careful observation of mice in their home cage can provide a wealth of information about the health and welfare of the animals. Activity, nest building, interaction with cage mates and general appearance are indicators of general health and well-being. Hands-on physical examination provides an assessment of the animal’s hydration, body condition, observable abnormalities, and the presence of palpable anomalies in the abdomen. We will outline details that will be not only useful to the investigator, but quick and easy to perform as well.
Several infectious agents have been identified over the years that have either adverse affects on animal health and/or research outcome. For this reason, most mouse colonies are maintained as Specific Pathogen Free (SPF), free of defined infectious agents. SPF status is generally monitored by exposing sentinel animals to dirty bedding of colony animals and testing the sentinel animals. Tremendous effort and expense is expended maintaining SPF colonies and it is critical that all staff entering colonies and handling animals understand disease transmission and the importance of good practices to prevent pathogens from being introduced or spread.
Laboratory mice develop a number of common clinical conditions which will be described, together with recommended treatments and suggested endpoints. For instance, dermatitis is a poorly understood condition that is common and problematic, especially in animals with a C57BL/6 background. Some congenital conditions such as hydrocephalus or microphthalmia are also seen with a C57BL/6 background. Behavioral differences exist between strains and lines of animals with some prone to fighting when males are co-housed. BALB/c, FVB and SJL strains are particularly troublesome in this regard although management practices can help reduce fighting.
The Guide is based on three principles: Replace, Reduce and Refine. Replace animals whenever possible, reduce the number to lowest possible that will produce accurate conclusions, and refine the experimental paradigm to improve the science and the care of the animal. These principles were introduced by British investigators William Russell and Rex Burch in 1959 in response to the moral and ethical concerns associated with the use of animals in research (Russell and Burch, 1959; Flexnell, 2002). Refinement is a principle that directly relates to the topic at hand, and can be as simple as adding palatable food on the cage floor or as sophisticated as utilizing telemetry to monitor physiology and activity.
The 8th Guide published in 2011 also emphasizes the refinement of end points and states that “The use of humane endpoints contributes to refinement by providing an alternative to endpoints that result in unrelieved or severe animal pain and distress.” In other words, an animal should be euthanized at the earliest possible point that will provide experimental data in order to minimize suffering. A variety of refined endpoints for multiple species have been published and they include data-based systems for assessing animals, drops in core body temperature as an alternative endpoint, endpoints for tumors and ascites production, and changing from an awake sepsis model to an anesthetized sepsis model, among others. (Hendriksen and Steen, 2000; Morton, 2000; Olfert and Godson, 2000; Minecci et al., 2007; Paster et al., 2009; Sass, 2000; Stokes, 2000; Stokes, 2002; Toth, 1997; Toth, 2000; Toth and Gardiner, 2000; Wallace, 2000).We discuss endpoints in mice for the consideration of the investigator.
COLONY HEALTH SURVEILLANCE
Colony health surveillance is typically part of the overall veterinary care program to ensure the SPF status of the animal facility. Many microbial outbreaks are subclinical in mice. Therefore, microbiological surveillance of colonies is required for detection and to ensure the appropriate health status of the colony and individual mouse. Sentinels are animals that are free of excluded microorganisms and are exposed to dirty bedding of colony animals to determine if excluded microorganisms are present in the colony animals. Health surveillance testing of sentinel and / or colony (non-sentinel) animals can include the following: gross, histopathology, and parasitology assessments, testing of serology samples for antibodies or antigens, culture or isolation of microorganisms, and molecular diagnostics such as the polymerase chain reaction (PCR) test. The diagnostic procedures utilizing samples collected from live animals for colony health surveillance include those used to assess the health of individual mice. For example, collection of: a fur sample for an ectoparasitology exam, fecal material for endoparasitology and PCR testing, and blood for serology testing can help determine the health status of an individual animal. Additional details regarding surveillance programs can be found in the text, Fox, et al., 2002. “Laboratory Animal Medicine,” 2nd ed. An example of commercial laboratory services for health surveillance can be found at:http://www.criver.com/enUS/ProdServ/ByType/ResAnimalDiag/Pages/home2.aspx.
Enormous expense is involved with maintaining SPF colonies. The protective personal equipment (PPE) used and the regular purchase and testing of sentinel animals is a significant investment by the facility/institution. Outbreaks of disease agents exponentially increase that cost. To contain the disease outbreak extensive additional testing is necessitated. For this reason facility staff and investigative staff should do their utmost to follow facility procedures, traffic patterns and standard operating procedures. For instance, in a facility where animals are changed in hoods using appropriate micro-isolator techniques then investigative staff should follow those same practices (e.g., not open cages outside of the hood). Another important consideration in this same vein is following appropriate procedures for introducing animals from outside facilities. Typically, there is a gatekeeper through who all imports of animals are managed. This is to ensure that their health status is known prior to arrival and confirmed after arrival before introduction into the main animal colony.
EXAMINATION AND ASSESSMENT OF THE MOUSE
An overall assessment of the health and welfare of a research mouse includes an evaluation of the animal in its home cage and a hands-on exam. Because mice are easily stressed by handling, the cage side exam should be performed first. Observing the mouse in its home cage will provide information about the animal’s overall appearance and activity level, the interaction with the environment, including nest building, and its behavior with respect to its cage mates. The hands-on examination allows assessment of observable abnormalities, hydration status, body condition, and the presence of abnormalities in the bones, genitals and abdomen.
Home cage evaluation
Mice are inquisitive and active and will generally be observed moving around the cage, grooming, eating, drinking and interacting with cage mates; particularly after being stimulated by having their cage picked up and moved from the shelf or rack.
Behavioral indicators of a welfare issue can be obvious including wounds and limping, hunched posture, dull or sluggish movements, large or open tumors, or a mouse that does not move when the cage is manipulated. Many behaviors are more subtle and non specific and will take practice and time to evaluate. Among these is nest building. All mice will build a nest if given suitable material (Hess et al., 2008). A mouse that is placed in a new cage with nesting material and has not built a nest by the next day warrants veterinary attention and possibly euthanasia. Similarly, the absence of feces in a cage after the mouse has been housed several hours suggests the animal has not been eating.
Mice are prey species and will generally mask signs of pain. If evaluating mice for pain after surgery or a procedure that has the potential to be painful, the observer needs to be quiet and monitor the mice without moving the home cage. Mice, like all animals express emotion including pain through facial expressions. Many of the expressions are identical to those expressed by humans in pain including squinting the eyes and contracting the skin around the nose and mouth. Mice may also pull their ears back (Langford et al., 2010;Figure 1). Mice in pain are less active than normal, although this type of qualitative assessment is difficult to evaluate on cage side exam unless it is severe (Clark et al., 2004; Roughan et al., 2009).
Figure 1.

Facial expressions in mice indicating pain and/or distress include squinted eyes, contracted skin around nose and ears pulled back.
Developing and assigning a study specific pain scale can be an effective tool to facilitate communication between observers and can serve as a convenient indication when an animal’s condition is deteriorating or has reached a clinical end point. Table 1 is an example of a general clinical pain score.
Table 1.
Assessing Pain and Distress in Mice
| PAIN AND DISTRESS ASSESSMENT |
EXAMPLES |
|---|---|
| 1) no indication of pain and distress | Normal; well groomed; alert; active; good condition; asleep or calm; normal appetite; BCS=3,4 or 5 |
| 2) mild or anticipated pain and distress | Not well groomed; awkward gait; slightly hunched; looks at wound or pulls away when area touched; mildly agitated; BCS=2 |
| 3) moderate pain and distress | Rough hair coat; dirty incision; squinted eyes; moves slowly; walks hunched and/or slowly; depressed or moderately agitated; slight dehydration; pruritic; restless; uncomfortable; not eating or drinking; BCS= 2-. |
| *4) severe pain and distress | Very rough hair coat; eyes sunken (severe dehydration); slow to move or non-responsive when coaxed; hunched; large abdominal mass; dyspnea; self mutilating; violent reaction to stimuli or when approached; BCS=1 |
Handling and Hands on evaluation
Laboratory mice are generally docile but will move quickly or jump away from the person trying to restrain them, and some strains may bite. Mice can be moved a short distance for examination by being picked up at the base of the tail and placed onto the top of their cage allowing the mouse to grasp the bars with the forefeet and direct its effort away from the handler. Very obese or pregnant mice should have a hand placed under the abdomen to prevent the heavy abdominal contents from compressing the diaphragm and limiting respiration. Retain control of the tail to prevent the mouse from escaping and potentially harming itself.
Restraint. With the animal restrained on top of the cage, run a finger over the animal’s coat to feel for wounds that may be covered with fur and feel for masses that may not have been obvious when the mouse was moving around the cage.
Hydration. Severely dehydrated mice will be weak and often will look paralyzed in their rear legs. These mice may also have trouble gripping the cage bars with their forefeet. Other symptoms of severe dehydration include sunken or recessed eyes and fuzzy facial fur, which results due to piloerection. More moderate dehydration can be detected by pinching the skin over the shoulder blades. In a well hydrated mouse the skin will quickly return to its original shape. The skin remaining bunched up is an indication of dehydration.
Body Condition. Assess body condition in mice by passing a finger over the sacroiliac bones (the spine and hip bones) and assign a score from one to five. A score of one indicates emaciation and score of five, obesity. In an optimally conditioned mouse, scored a three, the bones are palpable but not prominent. Figure 2 illustrates the appearance and the feel on palpation of the different levels of body condition (Foltz and Ullman-Cullere, 1999; Ullman-Cullere and Foltz, 1999). Body condition provides a more sensitive measure of welfare than body weight in the mouse because many common health conditions such as tumors can cause increases in body weight while breaking down body fat and muscle.
Figure 2.
Body Condition Scoring (BCS) is a quick, easy and reliable method for assessing mouse health. It utilizes a scoring system of 1 to 5 with 3 being the optimal condition, 1 being emaciated and 5 being obese.
Restraint and physical examination
While holding the animal by the tail with the little finger, use the forefinger and thumb to gently pin down the head and grasp the loose skin over the back of the neck. The remaining fingers can be used to scruff the skin along the back of the mouse, allowing the mouse to be picked up and restrained. Once restrained, examine the animal from its nose to the tail. Perform the exam in the same order each time an animal is examined to establish a pattern that will help prevent overlooking a more subtle abnormality.
Face and mouth. Evaluate the eyes, ears, face and neck for abnormalities. Examine the incisors by gently moving the lips using a cotton tipped applicator. Evaluate the color of the mucous membranes, which should be pink. The mucous membranes around the eyes and the skin of albinos appearing pale, bluish, or brick red in coloration indicate conditions such as anemia, hypoxia, or systemic infection and circulatory failure. These are near terminal conditions and indications for euthanasia. Note that a greenish hue may be due to genetic constructs with green fluorescent protein and are expected in some strains.
Feet and limbs. Next, examine the feet and limbs. Explore any gait abnormalities observed during the cage side exam. Lameness in one limb may be due to a wound on the foot or bony tumor that can be felt along the length of the bone. Pinch the toes to distinguish between weakness in the limbs and paralysis. Weak mice will generally pull away when the toes are pinched.
Genital abnormalities. Evaluate the female mammary chain for masses, abnormalities around the nipples or irregularities around the vulva. Examine the male penis by gently sliding back the prepuce. A purple or distended penis during exam likely indicates a urinary obstruction, which is a painful and life threatening condition. Check the rectal area for swelling, trauma or prolapsed tissue. Swellings adjacent to the rectum in older male mice likely indicate cysts in the reproductive glands and are generally benign.
Abdominal palpation. Palpate the abdomen by gently compressing the contents between the fingers from just under the ribs down to the hips. Common abnormalities palpated in the cranial aspect of the abdomen include tumors of the liver and spleen. In male mice, the most common abnormality felt in the middle part of the abdomen is due to enlargements of the kidneys secondary to urinary obstruction. In pregnant females, the distended uterus can be palpated in the mid abdomen and can extend up under the rib cage. In older females or retired breeders, masses in the mid abdomen are generally due to uterine tumors. The bladder can be felt in the caudal abdomen. Mice will generally urinate as a stress response when picked up. A large or distended bladder can indicate an obstruction. In males, the glands of the reproductive organs can become enlarged and distended with fluid as the mice age which can be palpated in the caudal abdomen.
-
Special consideration for post surgical mice. In the first one to five days after a surgical procedure, mice should be evaluated at least daily. Examine the surgical incision to ensure that it is intact and that the area is clean and dry. Excessive grooming in the area of the incision can be a sign of pain and may indicate that the incision has become infected. A mild amount of redness immediately around the incision is normal in the first two to three days, but excessive redness, a thick discharge, or swelling are symptoms of surgical site infection and warrant veterinary evaluation. The following link is to a post-op monitoring form that may be of use to the investigative and/or veterinary team.
See http://www.ors.od.nih.gov/sr/dvr/od/Documents/Post_Op_Form.docx
COMMON CLINICAL HEALTH CONDITIONS OF MICE
While mice are valuable research tools, they are also living animals with characteristics and health issues that may influence or be of concern while conducting a study. A holistic approach to evaluation of clinical conditions, including influences on the research and the welfare of the animals will allow the investigative and veterinary teams to make appropriate decisions. The discussion here will cover the most frequently seen conditions, but is not an exhaustive listing. The photographs included will illustrate many of these conditions. Treatment suggestions are based on the author’s clinical experience but should always be discussed with the veterinary team at your institution. Also, keep in mind that the physiology of the animal may be affected by responses to the condition itself and by any treatments administered. Finally, euthanasia may be the most appropriate response for both humane and research related reasons.
Skin lesions
Skin lesions are one of the most commonly observed clinical problems in mice and arise from many different causes. Often these can be clinically managed to keep the mouse on study or retain it for breeding, but strain predilections must also be considered. The immune system of the mouse will be activated to fight these lesions, which must be considered in light of the study protocol.
Fight wounds: Most commonly seen in co-housed male mice, especially from strains such as BALB/c, SJL, and FVB, however this behavior can also occur between females or in mixed sex groups. The typical presentation is a cluster of wounds on the rump, hips, and/or genital region, which may extend to the trunk of the body or forelegs. Often there is one aggressor in the cage which can be removed while beginning treatment of the other mice with antibiotics and analgesics systemically and/or topically. If the wounds are not severe, mice generally heal well. If wounds are severe, humane endpoint criteria should be discussed with your veterinary staff. Fighting can be minimized by housing only littermate males together, or single housing particularly aggressive strains.
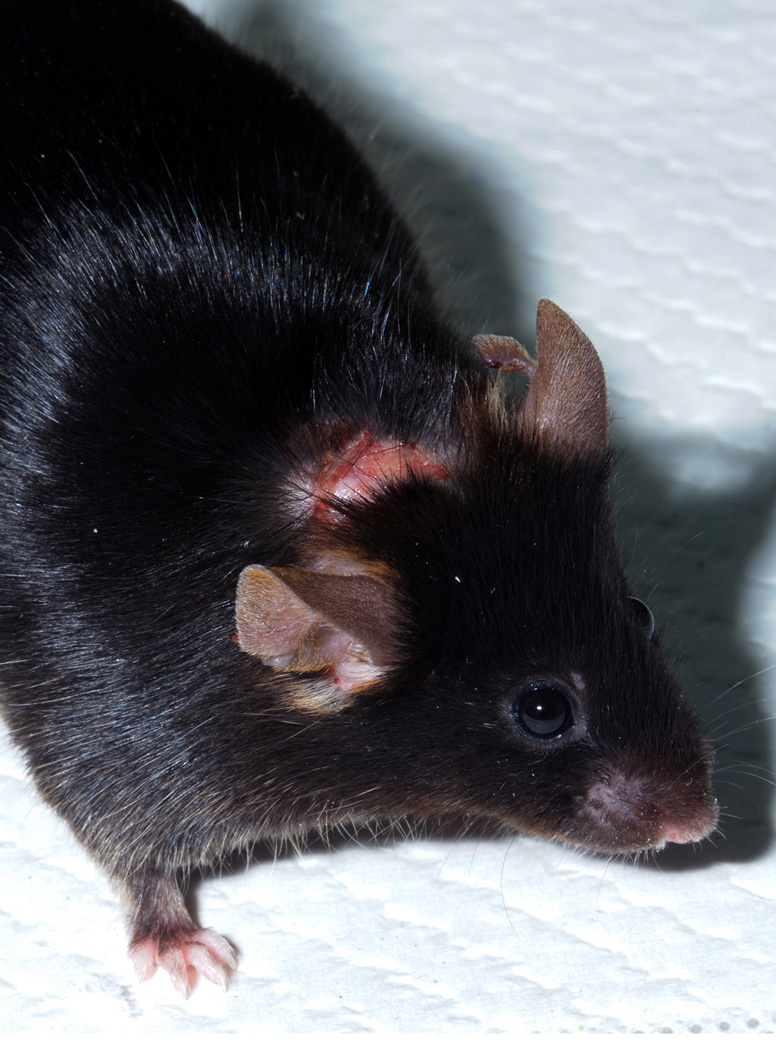
Ear dermatitis: Often related to ear tags used for identification. Although tags are normally very well accepted by the animals, problems may occur due to tag placement, sensitivity to the tag metals, or secondary to fighting. If irritation is noted, it is advisable to remove the tag if still present, and to use antibiotic and analgesic therapies to treat secondary infection and pruritis. Clipping of the hind toenails helps reduce trauma due to scratching.
Alopecia: Especially when seen in patches around the face or in one location on several mice within a group, is a sign of what is termed “barbering”. The skin is generally healthy, and short stubby hairs may be seen in the alopecic area. In some cases the whiskers or eyelashes may be missing. This condition is caused by over grooming by an animal’s cage mates or itself. It may be a dominance action or an obsessive compulsive grooming disorder. Unless there is secondary ulceration or inflammation of the skin, no medical treatment is necessary, but increased environmental enrichment has potential benefit.
Dermatitis: Includes ulcerative dermatitis (common in C57BL/6 background mice), miliary dermatitis, muzzle dermatitis involving the hair follicles, (referred to as furunculosis or botryomycosis), and contact dermatitis. Erosions of the skin or small raised scabbed lesions may be seen. If lesions are deep, large, or bleeding then aggressive care with analgesics, anti-inflammatory medications, and antibiotics, or euthanasia are indicated. For less severe lesions, control of infection by skin bacteria relieves the irritation and may allow healing; treatment may be systemic or topical. Many different treatment regimens have been used for ulcerative dermatitis including nutritional supplements and NK1 receptor antagonists (Lawson, G.W., et al. 2011).
Hyperkeratosis: Thickening of the skin without shedding of the surface dead epithelium is often a sign of irritation. It may also be a sign of neoplasia, bacterial skin infection, or mite infestation. Skin scrapings, fur plucks, or biopsy may be used diagnostically to guide treatment decisions. In nude mice Corynebacterium bovis has been noted to cause a flaky white dandruff-like skin condition which may be responsive to antibiotic treatment. Sterile caging is beneficial to reduce incidence.
Otitis: Head tilt or circling behavior can be associated with inflammation within the inner or outer ear. External lesions often respond to topical antibiotic, while inner ear conditions with nerve damage are more complicated and may lead to indications for euthanasia if the animal cannot maneuver to reach feed and water.
Tail lesions: May present as dermatitis, as fight wounds, or as concentric rings with hyperkeratosis. The latter has historically been related to very low humidity and is known as “ringtail”. Granulomas may form after tail biopsies for genotyping of mice. Treatments are the same as similar lesions found on the body. Small nicks used for collection of blood from the tail vein(s) will scab and generally heal without medical treatment.
Lumps and Bumps
“Lumps and Bumps” require further diagnostic evaluation to determine the cause and significance. Common causes are tumors (spontaneous or study induced), abscesses, cysts, lymphadenopathy, salivary gland hyperplasia, and reactions to injections especially if adjuvants are used.
Mammary tumors: A common problem in female mice which may be observed almost anywhere on the trunk of the body due to the extensive distribution of mammary tissue. These are subcutaneous, may be smooth or rough, and are usually easily moveable under the skin. In mice these tend to be malignant. Treatment is not advised.
Tumors: These should be evaluated with consideration of humane endpoints. Their size, location, secondary effects, etc. will be considered in decisions to observe, surgically remove, or recommend euthanasia.
Abscesses: Can occur in any location, but the most common are related to bite wounds, necrotic tumors, or blocked ducts to normal exocrine glands such as the preputial glands of male mice. They are usually a soft to firm swelling which may or may not be inflamed. Needle aspirate allows drainage of pus or caseous exudate which can be submitted for culture and antibiotic sensitivity testing to guide treatment plans. In some cases the abscess ruptures at the skin surface spontaneously. Treatments include draining and/or flushing the abscess, antibiotic therapy, and clean soft bedding if located ventrally. If not responsive to therapy, euthanasia must be considered.
Lymphadenopathy: Usually noted under the legs or in the neck region, but may occur anywhere lymph nodes are found, including within the abdomen. It may indicate a primary lymphoma or be an indication of systemic inflammatory responses. Evaluation of the inciting cause will help in determining treatment options or indications for euthanasia.
Reactions to injections: Seen with use of some adjuvants, result in small subcutaneous lumps at the local site, which may ulcerate to a small dry, open lesion in the skin. In most cases no treatment is necessary; however guidelines should be established in studies for treatment if these lesions become larger or deeper than expected. They are usually sterile, so the primary concern is to prevent secondary infection.
Eyes and surrounding tissues
Problems of the eyes and surrounding tissues are commonly seen in clinical evaluation of mice. These may involve the structures in front of the eye, eyelids, conjunctiva, tear production, or tear drainage; the eye itself including the cornea, lens, and deep structures; or the orbit behind the eye. Blepharospasm (squinting), discharge from the eye, or buphthalmia (bulging) are the most common initial presentations.
Microphthalmia or anophthalmia: Congenital conditions (common in C57BL/6) which present as a partially opened or closed eye. Often tear production continues with poor drainage resulting in a mild watery or waxy ocular discharge. Most mice are stable and groom to keep the area clean, precluding the need for treatment. Occasionally treatment for conjunctivitis may be needed.
Conjunctivitis: Presents as swollen pink to red tissue under the eyelid and often a thick ocular discharge. It may be caused by foreign bodies such as a piece of bedding or an aberrant eyelash, or be related to trauma to the conjunctiva or the globe. Often this can be treated by gentle flushing of the eye with a saline eye wash and topical application of an antibiotic lubricant ointment. Systemic antibiotics are also beneficial.
Keratitis: Inflammation of the cornea, presents as a cloudy or vascular surface of the eye, and is often combined with conjunctivitis. Lack of tear production or inability to close the eyelids properly can lead to drying of the corneal surface. Ulceration of the cornea may be secondary to drying, or a result of trauma or displacement of the lens of the eye. A dent in the cornea may be visible and a tissue plug may be present if all layers of the cornea have been penetrated. The cornea is well innervated, so corneal lesions have potential to be quite painful. Treatment with an analgesic either topically or systemically combined with lubrication and antibiotics leads to healing of most such lesions. In severe cases the globe may collapse or be lost; however healing of the orbit can occur and the mouse itself can be maintained.
Cataracts: Develop in some strains of mice, such as C57BL/6. They are seen as a central white material behind a clear cornea. Generally they do not cause any problem for the animal, however occasionally the lens will luxate leading to inflammation within the eye, glaucoma, or even expulsion of the lens. In such cases treatment is the same as for keratitis. White scars on the cornea may be confused with cataracts.
Retro-orbital tumors, blood clots, or abscesses: Result in bulging of the eye forward of the normal position, and often difficulty in closing the eyelids. These are difficult to treat, and euthanasia of the mouse is usually the preferred option.
Mobility issues
Mobility issues may be due to injuries, central nervous system disorders, or degenerative conditions.
Injuries:The most common include catching a foot or leg in some part of the cage apparatus and fight wounds. Each instance needs to be evaluated with the veterinary team for appropriate pain management, treatment, or euthanasia.
Neurologic conditions: Present in many ways including ataxia, head tilt, spinning when lifted by the tail, circling with inability to straighten out the path, and seizures. The primary clinical concern is ability to reach feed and water. Supportive care includes placing a soft diet on the floor of the cage, soft bedding, support of bodily functions such as urination, etc. Maintenance of such mice should be justified in the study proposal, or they should be euthanized.
Arthritis: Presents as swelling and often redness of joints, with favoring of the affected limb or a reluctance to move. This may be transient, related to the strain or study, or a result of trauma. Analgesia is usually indicated, and if pain is not resolved then euthanasia may be necessary.
Pododermatitis: Irritation on the bottoms of the feet may be part of a study model using foot pad injections, or may be caused by the floor of the cage (wire floors) or a wet cage. Treatment will depend on the specifics of the case but may include antibiotics, analgesics, and soft absorbent bedding.
Respiratory issues
Respiratory problems are seen as changes in the breathing pattern or nasal discharge. Signs of respiratory distress include dyspnea, shallow rapid breathing, gasping, or abdominal effort in breathing. Nasal discharge may be seen dried on the nostrils, or more commonly as crusty material on the forelegs from self grooming.
Immunosuppressed animals are highly susceptible to pulmonary infections similar to those seen in humans with AIDS. Use of sterile caging greatly reduces the risk for these animals. Preventive strategies are most effective; once symptoms develop there is little chance for successful treatment and euthanasia is the preferred option.
Congenital deformities
Congenital deformities are generally seen shortly after birth or at the time of weaning. These conditions may also lead to difficulty with pregnancy or pup delivery by the dam. If not part of the research model, it is best not to use affected mice or their parents for future breeding of a research line. These problems include:
Hydrocephalus: A condition in which fluid builds up in the ventricles of the brain and does not distribute normally between the brain and spinal cord. Visibly these mice have a large rounded head and shortened muzzle. They are smaller than littermates, and with time develop lethargy and neurologic abnormalities. Supportive care with special feed may be provided short term, but these animals rarely survive to adulthood. Euthanasia is the most humane option.
Malocclusion: Mouse teeth grow throughout life. The teeth should meet in such a way that they grind on each other and on the feed to keep the teeth a normal length. When this does not happen teeth may grow into the palate or out of the mouth making eating or drinking difficult for the animal. Deformities may be caused by congenitally defective jaw structure, damage to the developing teeth, or trauma to the mouth or jaw. Treatment short term involves trimming of the incisor teeth, however care for crooked or maloccluded teeth is a lifelong process. Euthanasia should be strongly considered and breeding is not recommended.
Runt pups: Very small, poorly developing pups usually indicate a genetic abnormality, or competitive disadvantage. In very large litters or if the dam is a poor milk producer, supportive care with soft dough or gel diets may provide sufficient support for runts to catch up to normal mice. However, if a dam produces runts in subsequent litters, it is best to retire her from breeding.
Imperforate vagina: A defect that may occur in the maturing young female mouse. It is produced by lack of opening of the vaginal membrane, and appears as swelling between the anus and genital papilla giving the appearance of a male mouse. Female mice have nipples while males do not, which helps in determining the sex of these mice. Although the vaginal canal can be surgically opened, breeding performance is usually poor, so this defect also is an indication for euthanasia.
Other common congenital deformities: Extra limbs or toes, lack of one or more limbs, an outwardly curved sternum (breast bone), small or absent eyes, abnormal organ development which may lead to difficulty breathing or a distended abdomen, closed rectum leading to inability to defecate, and others. Each should be evaluated by the veterinary staff with the investigator to determine the significance to the mouse, the line, and the best clinical approach.
Reproductive associated conditions
Reproductive-associated conditions are a common reason for veterinary care in mouse colonies. Clinical success requires rapid identification; thus observations by investigators as well as the animal care team can be very helpful in addressing these situations.
Dystocia: Difficulty in delivery of pups is one of the most common and clinically difficult conditions in mice. Signs of dystocia include a pup visible in the vaginal canal but not passing, immobility and dehydration, distension of the abdomen with little muscle tone, or labor for an extended period of time (more than a couple hours). Mice often deliver their pups during the night, so early morning health checks are the most common time to discover dystocia. Causes may include congenitally deformed or dead pups, very large pups, breach presentation, non-dilated vaginal canal, or exhaustion of the dam. A stuck pup may be removed by gently applying a lubricant around the pup, grasping the pup with gauze, and exerting gentle traction on the pup. However, if the mother is weak, she is unlikely to push out additional pups. Caesarian delivery of the pups and fostering to another breeder with pups the same age or slightly older is recommended for valuable lines. Provision of warm fluids subcutaneously, soft diets, and treatment with oxytocin and calcium may be of benefit.
Prolapse of vaginal or uterine tissue: May be secondary to vaginal hyperplasia, or excessive abdominal contractions. If minor, the exposed tissue may be cleaned, treated with a hyperosmotic solution to reduce swelling, and replaced via the vagina. A suture may be used to close the vaginal opening for a few days (mice have a separate external urethral opening so closure of the vagina temporarily is okay). However, if the amount of tissue is large or there is evidence of necrosis or self mutilation then euthanasia is indicated.
Prolapsed penis (paraphimosis): Occurs in male mice when the penis is not retracted into the surrounding prepuce. The usual presentation is a swollen, dragging penis, often with secondary trauma to the surface skin. Blockage of the urethra may also be noted. If the mouse is able to urinate, lubrication and placement on a soft bedding surface may allow the swelling to decrease and the penis to return to the normal position.
Perineal cysts: The bulbo-urethral glands may become filled with fluid giving the appearance of a severely enlarged scrotum or perineum. Needle aspiration yields a clear slightly yellow fluid. Generally no treatment is needed.
General conditions
Other general conditions that may be noted include:
Diarrhea: Noted as liquid feces when the animal is picked up or seen in the cage bedding. Diarrhea can lead to dehydration, so treatment is similar. Antibiotic therapy may also be beneficial.
Ascites: The buildup of fluid in the abdomen may be induced by a study; in which case the inclusion of endpoint guidelines is critical. It can also indicate organ failure of the heart or liver, neoplasia, or lymphatic malfunction. There is no long term treatment for this condition.
Anasarca: The buildup of fluid in the subcutaneous space, also indicative of organ failure, usually renal or lymphatic, is another indication for euthanasia. In examining a mouse this may initially be confused with obesity. Pitting edema, lethargy, and distribution of subcutaneous fluid will aid in differentiation of these conditions.
Rectal prolapse: A bulging of the distal colon out of the rectum, common in mice affected by Helicobacter spp. or intestinal parasites; but also caused by straining, constipation, or unspecific reasons. The health status of the colony will help in determining possible causes. Some respond well to hyperosmotic soaks, but many remain chronic and are managed by cleansing the exposed tissue, providing soft bedding, and in some cases using antibiotic and lubricant treatments as well.
Anorexia: While not directly observable, this is indicated when there is a lack of feces in a cage that has not just been cleaned, when there is no evidence of mice chewing on the chow, or when mice appear too thin or dehydrated. The first thing to check is the animal’s teeth for possible malocclusion then palpate for any masses in the abdomen and generally examine the mouse. Supportive care includes fluid therapy and feed on the floor.
Background characteristics
Background strain/stock characteristics include clinical presentations such as deafness, blindness, and hyperactivity. When choosing a research model or establishing a new genetically engineered line, these need to be considered. See Table 2.
Table 2.
Clinical Presentations Associated with Strain or Background
| Strain or Stock |
Predisposed to conditions |
|---|---|
| C57BL/6 | Hydrocephalus, Microphthalmia, Anopthalmia, Age related hearing loss, Malocclusion, Barbering, Ulcerative dermatitis |
| BALB/c | Male aggression, Heart ventricular mineralization, Corneal opacities, Conjunctivitis, Blepharitis, Periorbital abscesses, Age related hearing loss |
| C3H/He | Blindness, Corneal opacities, Age related hearing loss, Mammary tumors |
| FVB/N | Blindness, Seizures, Mammary hyperplasia (tumors rare), Hyperactivity |
| 129 | Blepharitis, Conjunctivitis, Megaesophagus |
| Swiss | Retinal degeneration, Amyloidosis |
| SJL/J | Blindness |
| A/J | Early hearing loss |
| DBA/2J | Audiogenic seizures, Early hearing loss |
Developed from information in: Hedrich, H.J., Bullock, G., and Petrusz, P., 2004; Percy, D.H., and Barthold, S.W., 2007
DEFINING AND REFINING ENDPOINTS
Important for refining the way we conduct animal research is identifying appropriate endpoints, and facilitating ways to adhere to those endpoints in practice. Endpoints are meant to minimize or eliminate pain or distress, when possible, and enhance animal well being. The Guide defines a humane endpoint as “(T)he point at which pain or distress in an experimental animal is prevented, terminated or relieved” (National Research Council, 2011).
Frequent health evaluations may be required to identify animals approaching a study’s endpoint, and these observations play an important role in assuring humane animal research. In some instances, the endpoint described in animal study proposals is a compromise between the humane endpoint and the experimental endpoint, the time at which scientific aims and objectives are met (National Research Council, 2011). The Principal Investigator, in collaboration with a veterinarian and the Institutional Animal Care and Use Committee (IACUC), is responsible for identifying a study endpoint that is both scientifically relevant and humane before animal studies commence(Morton and Griffiths, 1985).
Morbidity endpoints are preferred and considered more humane than moribundity or death endpoints because they allow interventions or treatments that prevent pain and distress. However, in cases when veterinary intervention interferes with experimental results, moribundity/death as an endpoint may be required to reach experimental goals. Some examples of scientifically justified moribundity/death models include: metastatic tumor models, infectious disease/vaccine challenge, pain modeling, trauma, production of monoclonal antibodies, toxicology, organ/system failure, and cardiovascular shock (National Research Council, 2011).
Identifying Animals Nearing Endpoint
Multiple criteria may indicate an animal has reached its study endpoint. While it is possible to describe an exhaustive list, Table 3 presents commonly referenced categories and criteria that can be used when planning a study and deciding on an appropriate study endpoint. It is not uncommon for an animal to show multiple clinical signs listed in the protocol while remaining below a study’sdescribed endpoint. In such cases, evaluation of the five criteria of an animal's condition as described by Morton and Griffiths is useful. These are: body weight, physical appearance, measurable clinical signs, unprovoked behavior and response to external stimuli (Morton and Griffiths,1985). These criteria, when considered with objective measurements and in consultation with a veterinarian, can help identify animals at the earliest endpoint. It is also important to note that clinical signs may arise spontaneously that are not described in an approved study protocol. Attending veterinarians may, based on the animal’s overall condition, consider an animal to be at its humane endpoint and recommend euthanasia.
Table 3.
Clinical Signs and Evaluation Criteria Used to Determine Humane Endpoints
| Clinical Signs (NIH, ARAC, 2011) |
Clinical Observations in Cancer Research and Toxicological Studies (Montgomery, 1990) |
|---|---|
|
|
|
Neoplasia Endpoints (NIH, ARAC, 2011) |
Clinical Signs of Moribundity (NIH ARAC, 2011) |
|
|
Special Considerations of Endpoints
Endpoint evaluation criteria presented in Table 3 is a good foundation for evaluating laboratory mice. Some conditions, such as aging studies warrant extra consideration when defining study endpoints. Aging mice often exhibit a host of clinical signs that would indicate disease in younger mice (Office of Animal Care and Use, National Institutes of Health, 2011), including decreased body condition, increased respiratory rate, and pallor; but these may be considered normal in an aging mouse. More subjective criteria, such as quality of life and general health may be used.
Genetically Modified Animals (GMA) is an example of studies where unexpected experimental outcomes may occur. Small pilot studies and additional screening/monitoring are recommended since the effects of the genetic modifications can be largely unknown (Stokes, 2000).One should also consider the use of body condition scores, rather than body weight, in the determination of endpoints in models involving tumors, ascites, and other diseases that cause organomegally. These conditions all can falsely increase body weight, despite concurrent muscle wasting and cachexia.
Importance of Training and Monitoring
Adequate training of staff to recognize clinical signs and identify animals at endpoint is key to minimizing pain and distress. When the nature or severity of an animal’s condition is in question, consultation with a veterinarian is useful not only in determining a diagnosis but in training oneself and the staff to recognize various disease states. Additionally, there are numerous training resources available to help staff become familiar with these clinical signs, such as the Charles River Handbook of Clinical Signs in Rodents and Rabbits (Pritchett-Corning, 2010).
The use of study specific records that detail both the assessment criteria for endpoints as well as the animal’s condition can play a key role in the effective monitoring of animals. Such a document would ideally include: the definition of the study endpoint and assessment criteria, frequency of observation, and the response required when the animal has reached the study endpoint (Office of Animal Care and Use, National Institutes of Health, 2011). Animal users and animal care staff can communicate more efficiently, and animals can be identified for intervention/euthanasia at the earliest possible time point, preventing or alleviating unnecessary pain or distress--a refinement by definition.
In summary, when we choose endpoints thoughtfully and use trained staff to monitor animals frequently, we improve the welfare of laboratory mice by minimizing pain or distress. Using training resources, key references on endpoints, and study-specific monitoring records lend to our mission of refining the way we do research on animals.
Figure 3.
Fight wounds. (A) Typical pattern of miliary wounds on the side of the body. (B) Deep wound on the caudal portion of the rump. (C) Wounds and associated swelling on the tail.
Figure 4.
Ear Dermatitis. Crusty lesions on and below the ear associated with loss of an eartag.
Figure 5.
Barbering. (A) Thin coat with short stubby hairs on the head and neck. (B) Two mice, one with minor and one with extensively barbered fur. Note that the skin is healthy in these cases.
Figure 6.
Ulcerative dermatitis, deep ulcerative lesion with redness and moist surface at the base of the neck.
Figure 7.
Ringtail. Circular constriction is noted with normal skin coloration. There may be one ring as shown or a series of rings around the tail.
Figure 8.
Tumor. A subcutaneous irregular mass is shown caudal to the front leg of this nude mouse.
Figure 9.
Conjunctivitis. The left eye demonstrates swelling of the eyelids, redness of conjunctiva, and serous discharge. Compare to the normal right eye.
Figure 10.
Keratitis. The left cornea is white, opaque, and dry. Compare to the normal right eye.
Figure 11.
Retrobulbar abscess. Swelling and discoloration are noted below and caudal to the orbit of the left eye. Compare to the normal right eye.
Figure 12.
Arthritis. The mouse on the left has swelling of the hock (ankle) joint associated with arthritis. A normal mouse is shown on the right.
Figure 13.
Malocclusion. The incisor teeth are unequal in length and the shorter tooth angles inward more than normal. Teeth may be observed to grow inward or outward, and may be very curved or broken as shown.
Figure 14.
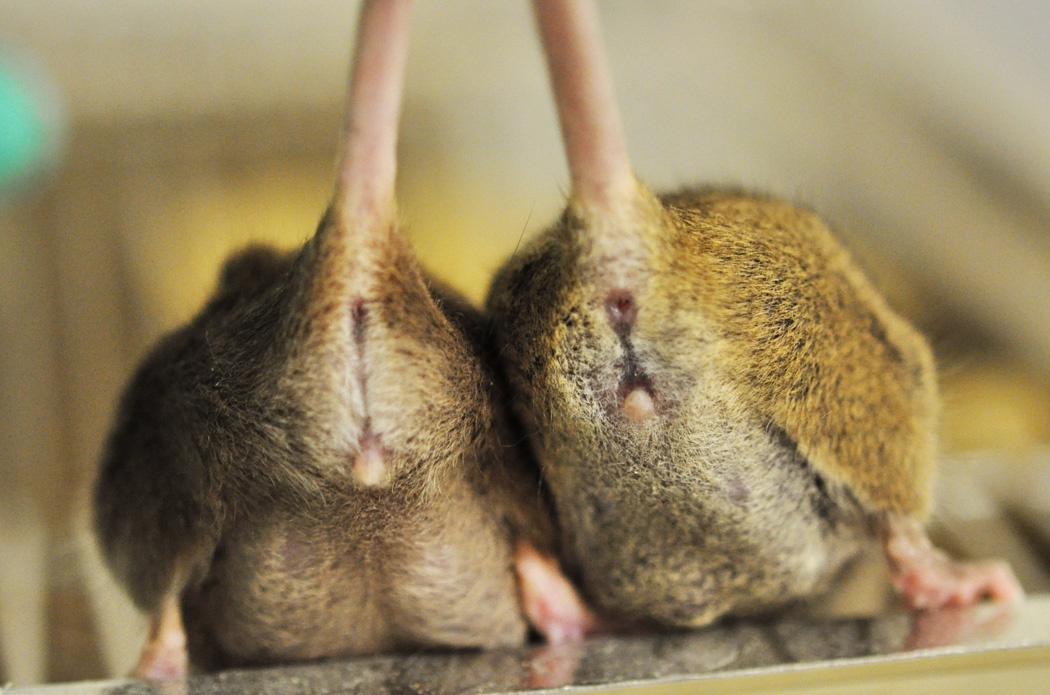
Runts. A runt or small mouse is shown on the left compared with its normal littermate on the right.
Figure 15.
Imperforate vagina. Both are female mice. The mouse on the left lacks the normal vaginal opening and shows subcutaneous perineal swelling due to accumulated secretions which are unable to drain. Normal mouse is shown on the right.
Figure 16.
Bulbourethral gland cyst. Swelling in the right side of the scrotum is caused by a cystic bulbourethral gland. This can be confirmed by performing a needle aspiration, with fluid indicating a cyst while no fluid may be indicative of a tumor.
Figure 17.
Ascites. Fluid accumulation within the abdomen leads to a potbellied appearance with prominent spine.
Figure 18.
Rectal prolapse. Red, edematous mucosal tissue is seen bulging from the rectal orifice.
Footnotes
Internet Resources with Annotations:
http://phenome.jax.org Mouse phenome database maintained by The Jackson Laboratory with detailed phenotype strain survey data. The Jackson Laboratory site www.jax.org has links to many valuable resources such as mouse nomenclature, resource manuals, and specific strain information.
http://www.ors.od.nih.gov/sr/dvr/od/Documents/Post_Op_Form.docx A link to a post-op monitoring form that may be useful to either the investigative team and/or the veterinary team.
An example of commercial laboratory services for health surveillance can be found at: http://www.criver.com/en-US/ProdServ/ByType/ResAnimalDiag/Pages/home2.aspx
VIDEOS
Video 1
File name: Mouse Health Evaluation Video.mov
Title: “Health Evaluation of Experimental Laboratory Mice”
Legend: Instructional video detailing normal behavior, home cage observation, restraint, body conditioning, and physical examination of experimental laboratory mice.
Video 2
File name: Circling in Laboratory Mice.mov
Title: “Circling in Laboratory Mice”
Legend: An example of circling in a laboratory mouse.
Video 3
File name: Ataxia in Laboratory Mice.mov
Title: “Ataxia in Laboratory Mice”
Legend: An example of ataxia in a laboratory mouse.
LITERATURE CITED
- Clark MD, Krugner-Higby L, Smith LJ, Heath TD, Clark KL, Olson D. Evaluation of liposome-encapsulated oxymorphone hydrochloride in mice after splenectomy. Comp. Med. 2004;54(5):558–563. [PubMed] [Google Scholar]
- Flecknell P. Replacement, reduction and refinement. ALTEX. 2002;19(2):73–78. [PubMed] [Google Scholar]
- Foltz CJ, Ullman-Cullere M. Guidelines for Assessing the Health and Condition of Mice. Lab Animal. 1999;28:28–32. [PubMed] [Google Scholar]
- Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory Animal Medicine. 2nd ed. San Diego: Academic Press; 2002. [Google Scholar]
- Hendriksen CFM, Steen B. Refinement of vaccine potency testing with the use of humane endpoints. ILAR J. 2000;41:105–113. doi: 10.1093/ilar.41.2.105. [DOI] [PubMed] [Google Scholar]
- Hess SE, Rohr S, Dufour BD, Gaskill BN, Pajor EA, Garner JP. Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. J. Am. Assoc. Lab. Anim. Sci. 2008;47:25–31. [PMC free article] [PubMed] [Google Scholar]
- Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, LaCroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, tabaka JM, Wong D, van den Maagdenbert AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain the laboratory mouse. Nat Methods. 2010;7(6):447–450. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- Lawson GW, Sato A, Fairbanks LA, Lawson PT. Vitamin E as a treatment for ulcerative dermatitis in C57BL/6 mice and strains with a C57BL/6 background. Cont. Top. 2005;44(3):18–21. [PubMed] [Google Scholar]
- Minecci PC, Deans KJ, Hansen B, Parent C, Romines C, Gonzales DA, Ying S, Munson P, Suffredini AF, Feng J, Solomon MA, Banks SM, Kern SJ, Danner RL, Eichacker PQ, Natanson C, Solomon SB. A canine model of septic shock: Balancing animal welfare and scientific relevance. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H2487–H2500. doi: 10.1152/ajpheart.00589.2007. [DOI] [PubMed] [Google Scholar]
- Montgomery CA. Oncological and toxicological research: Alleviation and control of pain and distress in laboratory animals. Cancer Bulletin. 1990;42:230–237. [Google Scholar]
- Montgomery CA. Control of animal pain and distress in cancer and toxicologic research. J. Am. Vet. Med. Assoc. 1987;191(10):1277–1281. [PubMed] [Google Scholar]
- Morton DB. A systematic approach for establishing humane endpoints. ILAR J. 2000;41:80–86. doi: 10.1093/ilar.41.2.80. [DOI] [PubMed] [Google Scholar]
- Morton DB, Griffiths PH. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- National Research Council. The Guide for the Care and Use of Laboratory Animals. 8th Ed. Washington, D.C.: National Academies Press; 2011. http://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf. [PubMed] [Google Scholar]
- Office of Animal Care and Use, Animal Research Advisory Committee, National Institutes of Health. Guidelines for Endpoints in Animal Study Proposals. http://oacu.od.nih.gov/ARAC/documents/ASP_Endpoints.pdf.
- Olfert ED, Godson DL. Humane endpoints for infectious disease animal models. ILAR J. 2000;41:99–104. doi: 10.1093/ilar.41.2.99. [DOI] [PubMed] [Google Scholar]
- Paster EV, Villines KA, Hickman DL. Endpoints for mouse abdominal tumor models: Refinement of current criteria. Comp. Med. 2009;59:234–241. [PMC free article] [PubMed] [Google Scholar]
- Roughan JW, Wright-Williams SL, Flecknell PA. Automated analysis of postoperative behavior: assessment of HomeCageScan as a novel method to rapidly identify pain and analgesic effects in mice. Lab Animal. 2009;43:17–26. doi: 10.1258/la.2008.007156. [DOI] [PubMed] [Google Scholar]
- Russell WMS, Burch RL. The principles of humane experimental technique. London: Methuen and Co.; 1959. (Reissued: 1992, University Federation for Animal Welfare, Herts, UK). [Google Scholar]
- Sass N. Humane endpoints and acute toxicity testing. ILAR J. 2000;41:114–123. doi: 10.1093/ilar.41.2.114. [DOI] [PubMed] [Google Scholar]
- Stokes WS. Reducing unrelieved pain and distress in laboratory animals using humane endpoints. ILAR J. 2000;41:59–61. [Google Scholar]
- Stokes WS. Humane endpoints for laboratory animals used in regulatory testing. ILAR J. 2002;43:S31–S38. [PubMed] [Google Scholar]
- Toth LA. The moribund state as an experimental endpoint. Contem. Top. Lab. Anim. Sci. 1997;36:44–48. [PubMed] [Google Scholar]
- Toth LA. Defining the moribund condition as an experimental endpoint for animal research. ILAR J. 2000;41:72–79. doi: 10.1093/ilar.41.2.72. [DOI] [PubMed] [Google Scholar]
- Toth LA, Gardiner TW. Food and water restriction protocols:Physiological and behavioral considerations. Contemp. Top. Anim. Sci. 2000;39:9–17. [PubMed] [Google Scholar]
- Ullman-Cullere M, Foltz CJ. Body Condition Scoring: A Rapid and Accurate Method for Assessing Health Status in Mice. Lab. Anim. Sci. 1999;49:319–323. [PubMed] [Google Scholar]
- Wallace J. Humane endpoints and cancer research. ILAR J. 2000;41:87–93. doi: 10.1093/ilar.41.2.87. [DOI] [PubMed] [Google Scholar]
- Williams-Fritze MJ, Carlson-Scholtz JA, Zeiss C, Deng Y, Wilson SR, Franklin R, Smith PC. Maropitant citrate for treatment of ulcerative dermatitis in mice with a C57BL/6 background. J. Am. Assoc. Lab. Anim. Sci. 2011;50(2):221–226. [PMC free article] [PubMed] [Google Scholar]
Key References with Annotations
- Hedrich HJ, Bullock G, Petrusz P. The Laboratory Mouse. San Diego, CA: Elsevier Academic Press; 2004. . This book contains excellent introductory information in first few chapters with strain characteristics in Chapter 3. Detailed systems information excellent as a reference.
- Percy DH, Barthold SW. Pathology of Laboratory Rodents and Rabbits. Third edition. Ames, IA: Blackwell Publishing; 2007. . The first chapter of this book contains excellent general pathology information on laboratory mice, including strain characteristics.
- Pritchett-Corning KR, Girod A, Avellaneda G, Fritz PE, Chou S, Brown MJ. Handbook of Clinical Signs in Rodents and Rabbits. Charles River Laboratories; 2010. www.criver.com.. This is a pictorial guidebook aimed at animal care personnel, organized by anatomic and organ system sections which has additional information on rodent clinical observations.