Figure 2. Schematic diagram of SCN paracrine positive-feedback coupling from a single neuron perspective.
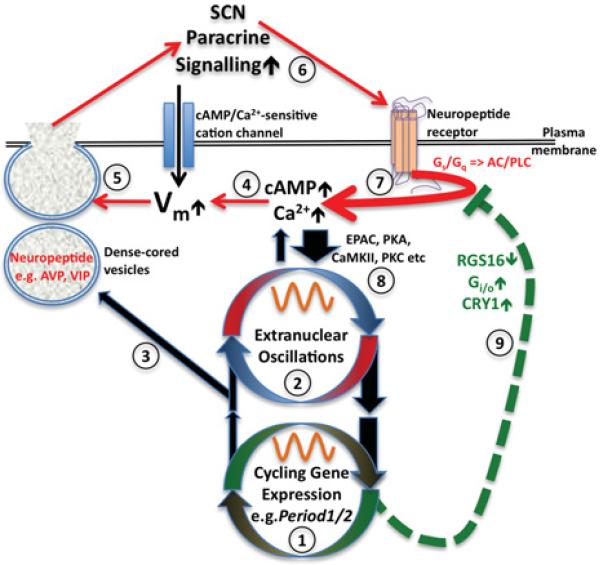
Cellular timekeeping results from reciprocal cross-talk between transcriptional-translational feedback loops (1) with extranuclear oscillations (2) in signalling and metabolism to facilitate rhythmic regulation of CCGs (3), e.g. AVP, and also increased cAMP/Ca2+ signalling around anticipated dawn (4). cAMP/Ca2+ depolarize resting membrane potential (Vm), thereby increasing electrical activity and neuropeptide release (5), further elevating cAMP/Ca2+ signalling within the network, eliciting further neuropeptide release (6). Neuropeptide receptor activation amplifies cAMP/Ca2+ signalling (7), and activates downstream effectors (8). Later in the day, cAMP/Ca2+ signalling decreases through neuropeptide depletion and/or receptor desensitization/internalization and/or change in gene expression of inhibitors of G-protein signalling (9). At night, cAMP/Ca2+-signalling, and therefore electrical activity, is reduced. CaMKII, Ca2+ /calmodulin-dependent protein kinase II; EPAC, exchange protein directly activated by cAMP; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C.
