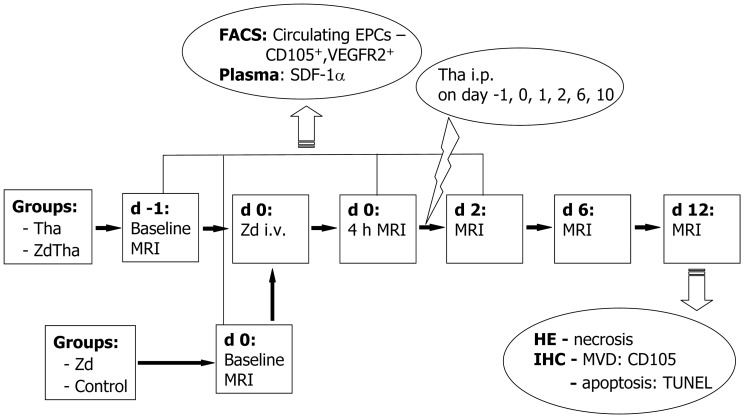
Figure 1. Study design.
Rats were randomly assigned into the following 4 groups: (group 1) Zd alone (n = 11); (group 2) Tha alone (n = 11); (group 3) ZdTha (n = 12); (group 4) untreated controls (n = 10). For groups 2 and 3, Tha (200 mg/kg) was injected intraperitoneally (i.p.) six times, on days (d) -1, 0, 1, 2, 6, and 10. For groups 1 and 3, Zd (50 mg/kg) was injected once intravenously (i.v.) on day 0. The controls were injected on days 0, 1, 2, 6, and 10 with the vehicles (solvents) of both agents (i.v. and i.p.) at the same time points that the other groups were injected. For all groups, MRI was performed before, and at 4 h, 2 d, 6 d, and 12 d after the initial treatment. Before the baseline, 4 h, and 2 d MRIs, rat tails were incised to collect blood samples for monitoring the levels of circulating EPCs and plasma SDF-1α in both Zd and ZdTha groups. At the end of the experiment, animals were sacrificed for histopathology examinations. (Zd, Zd6126; Tha, Thalidomide; ZdTha, Zd6126+Thalidomide; i.p., intra-peritoneal injection; i.v., intravenous injection; MRI, Magnetic resonance imaging; FACS, Fluorescence-activated cell sorting; EPCs, endothelial progenitor cells; VEGFR, vascular endothelial growth factor receptor; SDF-1α, stromal cell-derived factor-1α; HE, hematoxylin-eosin staining; IHC, Immunohistochemistry; MVD, microvessel density; TUNEL, terminal deoxynucleotidyl transferase biotin dUTP nick end labeling).