Figure 1. Assay for interactions between αB crystallin and HbA or HbS.
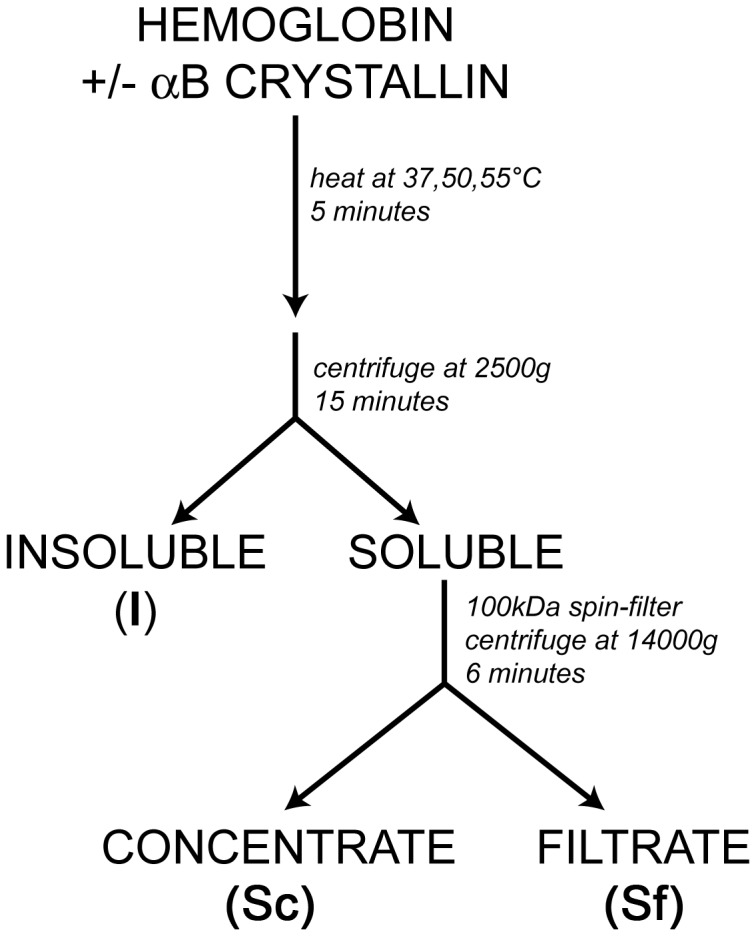
Samples of Hemoglobin A or Hemoglobin S were heated in the presence and absence of αB crystallin and then the interactive aggregates or bound complexes were separated by centrifugation. At low speed (2500 g) only the largest aggregates were in the insoluble (I) fraction, and the Hb bound to αB crystallin remained soluble in the supernatant (soluble) fraction. The soluble fraction was centrifuged again at 14,000 g using a 100 kDa spin-filter to separate the HbA or HbS bound to αB crystallin in the soluble concentrate (Sc) from the soluble HbA or HbS in the filtrate (Sf). With thermal destabilization, aggregation was expected to increase the amount of αB crystallin bound HbA or HbS in the insoluble (I) and soluble concentrate (Sc) fractions and decrease the amount in the soluble filtrate (Sf) fraction. The centrifugation experiments were conducted under conditions of minimal thermal stress to determine the sensitivity of αB crystallin to the earliest stages of protein unfolding and aggregation.
