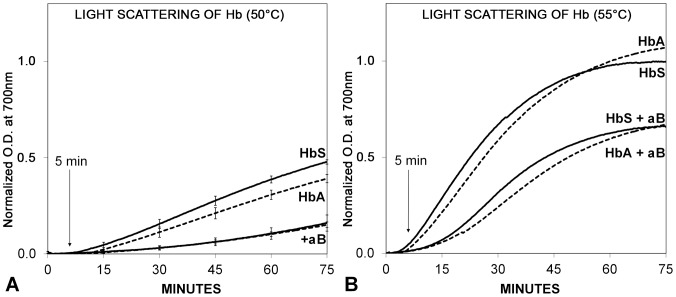
Figure 4. Thermal unfolding and aggregation of HbA or HbS in the presence or absence of αB crystallin.
Aggregation was initiated by heating the sample to 50°C (A) or 55°C (B). A. In the absence of αB crystallin, light scattering was measured as optical density (O.D.) in milliabsorbance units (mAU) and normalized to the maximum observed at 55°C. The light scattering increased progressively with increasing protein unfolding and aggregation at 50°C. There was a measurable inhibition of aggregation of unfolding HbA or HbS in the presence of αB crystallin. B. At 55°C the protective activity of αB crystallin on thermal unfolding and aggregation of HbA and HbS was more obvious. The difference in light scattering for HbA and HbS in the presence or absence of αB crystallin was not statistically significant. No increase in light scattering was observed at 37°C (not shown) which was consistent with the absence of a change in the UVCD (Fig. 3). Aggregation and light scattering of unfolding HbA and HbS were minimal at 5 minutes which was consistent with the conformation measured using UVCD in Fig. 3. At all temperatures, αB crystallin inhibited the aggregation of unfolding HbA and HbS measured using light scattering.