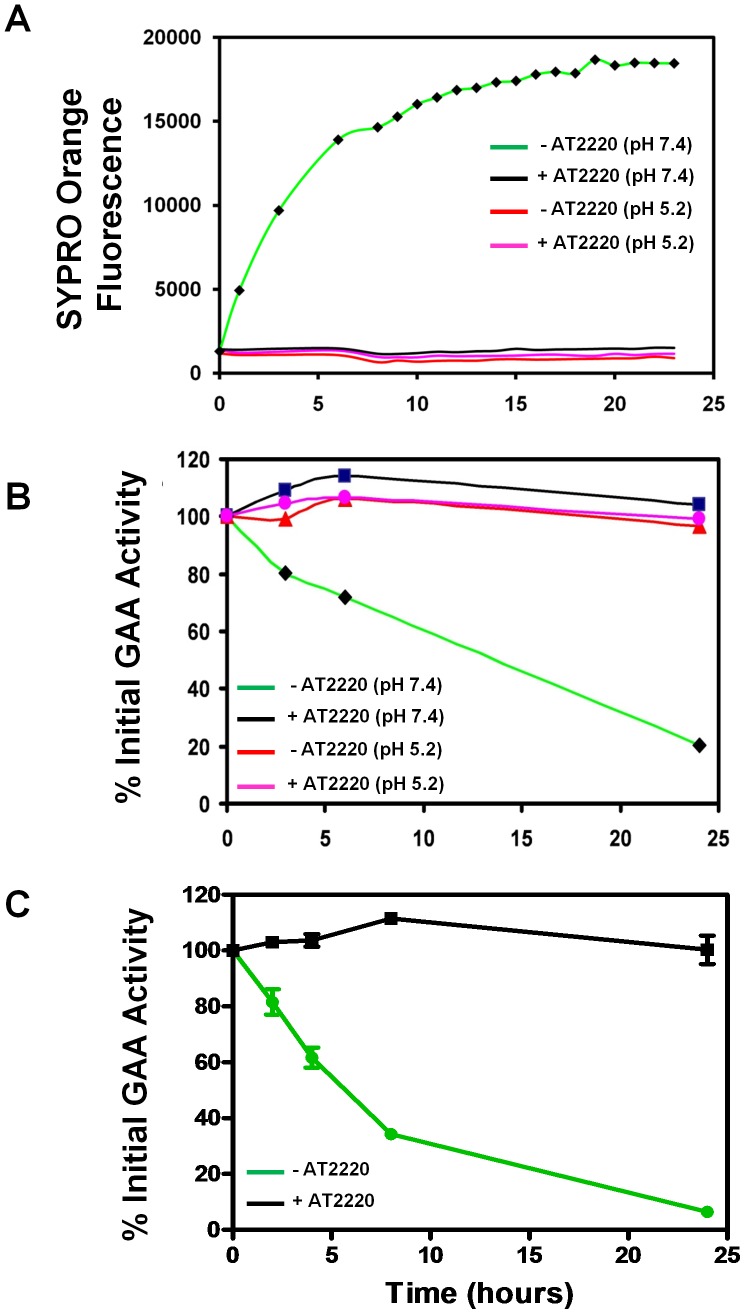
Figure 1. AT2220 increases the physical stability of rhGAA in vitro.
(A) Time course of rhGAA denaturation in neutral and acidic buffer at 37°C in the absence and presence of 50 µM AT2220. Denaturation was monitored by changes in the fluorescence of SYPRO Orange as a function of time. (B) Time course of rhGAA inactivation (i.e. loss of activity) in neutral and acidic buffer at 37°C in the absence and presence of 50 µM AT2220. (C) Time course of rhGAA inactivation (i.e. loss of activity) in human whole blood at 37°C in the absence and presence of 50 µM AT2220. In both (B) and (C), GAA enzyme activity was determined at the indicated time points using the fluorogenic substrate 4-MUG. To obtain relative enzyme activity levels, measurements at the various time points were compared to the activity at the zero time point.