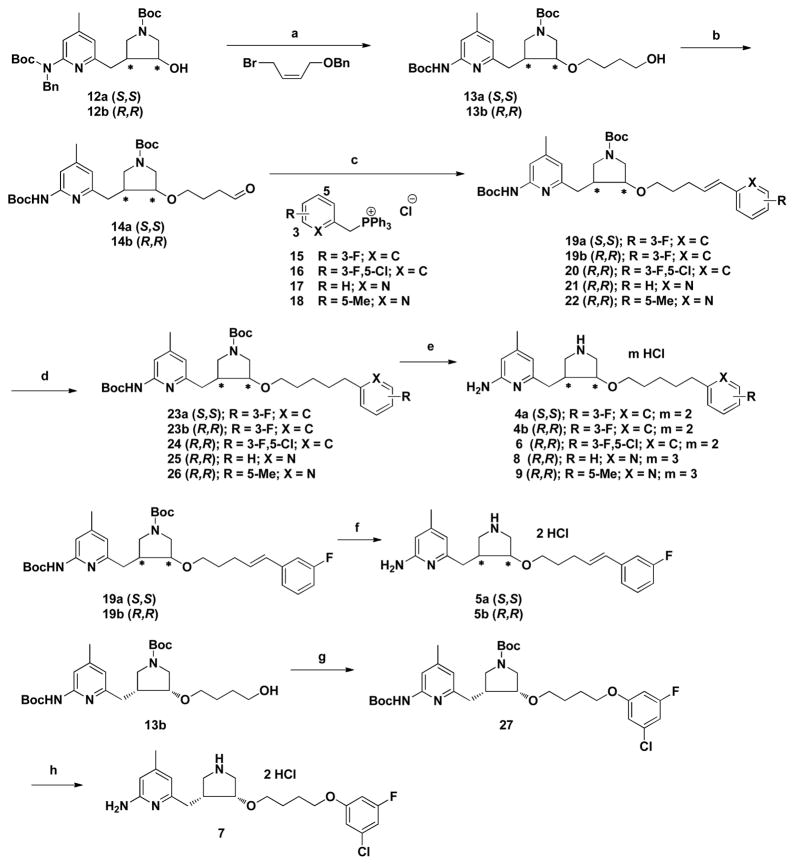
Scheme 1.
Synthesis of 4–9a
a Reagents and conditions: (a) (i) NaH, DMF, 0 °C, 3 h, (ii) 20 wt % Pd(OH)2/C, H2, EtOH, 60 °C, 36 h, 91–94%; (b) Dess-Martin periodinane, CH2Cl2, rt, 18 h, 89–92%; (c) (i) LHMDS, −78 °C to rt, THF, 8 h; (d) 10% Pd/C, H2, MeOH, rt, 12 h; (e) 3N HCl/MeOH, rt, 24 h, 47–69% for three steps; (f) 3N HCl/MeOH, rt, 24 h, 90–93%; (g) (i) PPh3, CBr4, CH2Cl2, 0 °C, 3 h, (ii) 3-chloro-5-fluorophenol, K2CO3, acetone, reflux, 12 h, quantitative; (h) 3N HCl/MeOH, rt, 24 h, quantitative.