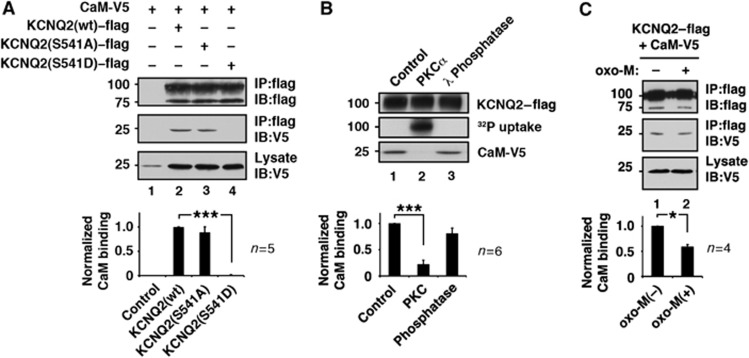
Figure 2.
PKC phosphorylation interferes with KCNQ2–calmodulin interaction. (A) Calmodulin binding to KCNQ2 protein and its PKC site mutants. ***<0.001 by nonparametric ANOVA followed by Dunn’s multiple comparisons test. (B) In-vitro phosphorylation of KCNQ2 protein by purified PKCα and its calmodulin binding capability. Phosphorylated KCNQ2 protein cannot bind calmodulin. Calmodulin binding of untreated control and dephosphorylated KCNQ2 are also shown. ***<0.001 by nonparametric ANOVA followed by Dunn’s multiple comparisons test. (C) Oxo-M treatment reduced calmodulin binding to KCNQ2. *<0.05 by paired t-test. Error bars indicate s.e.m. Figure source data can be found with the Supplementary data.