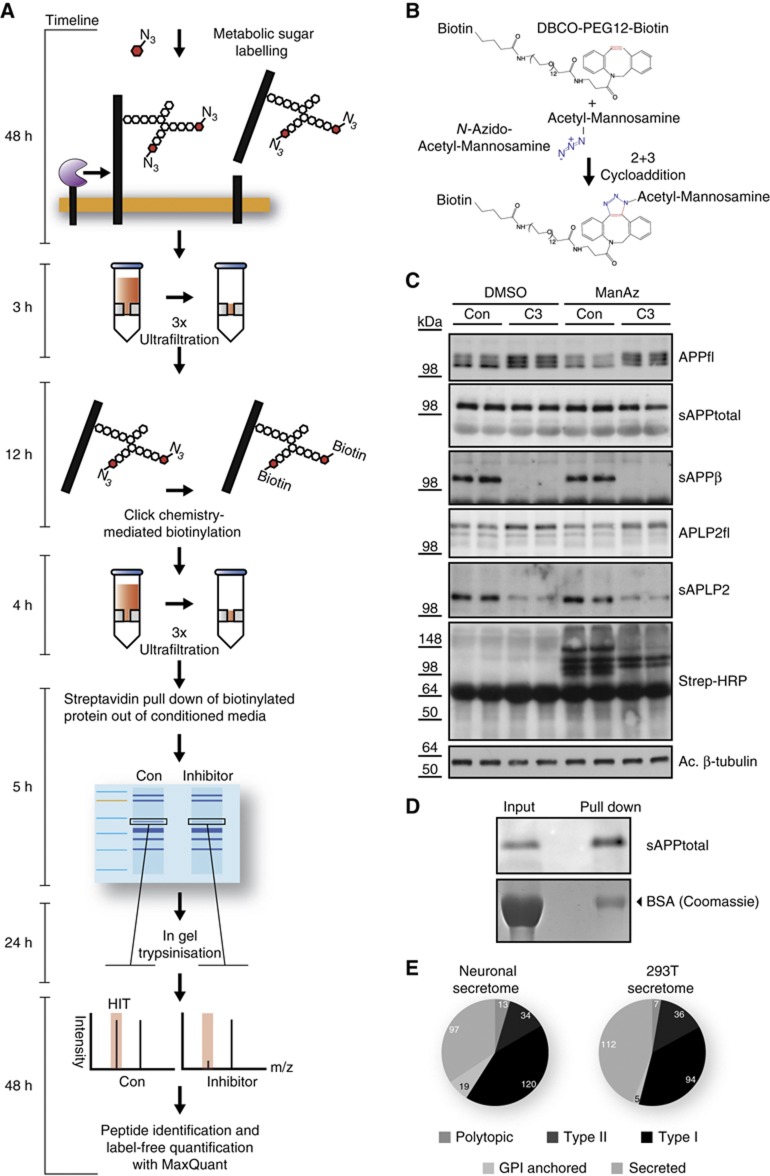
Figure 1.
Overview and validation of SPECS method. (A) Detailed description of the work flow of the SPECS method including a timeline. (B) Schematic representation of the click reaction between the azide group (blue) of 1,3,4,6-acetyl-N-acetyl-azido-mannosamine (ManNAz) and the strained alkyne (red) of dibenzylcycloocytne-PEG12-Biotin. (C) APP and APLP2 shedding were analysed in the presence of the BACE1 inhibitor C3 and ManNAz or DMSO as a control. Subsequently, click-chemistry reaction was performed in the conditioned medium. Full-length APP (APPfl) and APLP2 (APLP2fl) in the lysates as well as secreted APP and APLP2 (sAPPtotal, sAPPβ, sAPLP2) were detected by immunoblot. The analysis shows no significant changes of the shedding of APP and APLP2 upon addition of ManNAz. Biotinylated proteins (specific bands and broad smear) were detected with Streptavidin-HRP only when ManNAz was present. Acetylated (Ac) tubulin serves as a loading control. (D) Purification of glycoproteins by streptavidin pull down after click-chemistry reaction. Left: aliquot of conditioned medium is directly loaded (input). Right: upon streptavidin pull down, sAPPtotal is enriched by about two-fold, whereas serum albumin in the coomassie gel is reduced over 50-fold, leading to a specific enrichment of the glycosylated sAPPtotal by over 100-fold. (E) Distribution of glycoprotein types among all glycoproteins identified in the HEK293T (293T) secretome and the neuronal secretome. Only such proteins were included, which were detected by at least two peptides. Detailed listing of identified proteins and their topology is in Supplementary Tables 1, 2, 5 and 6.