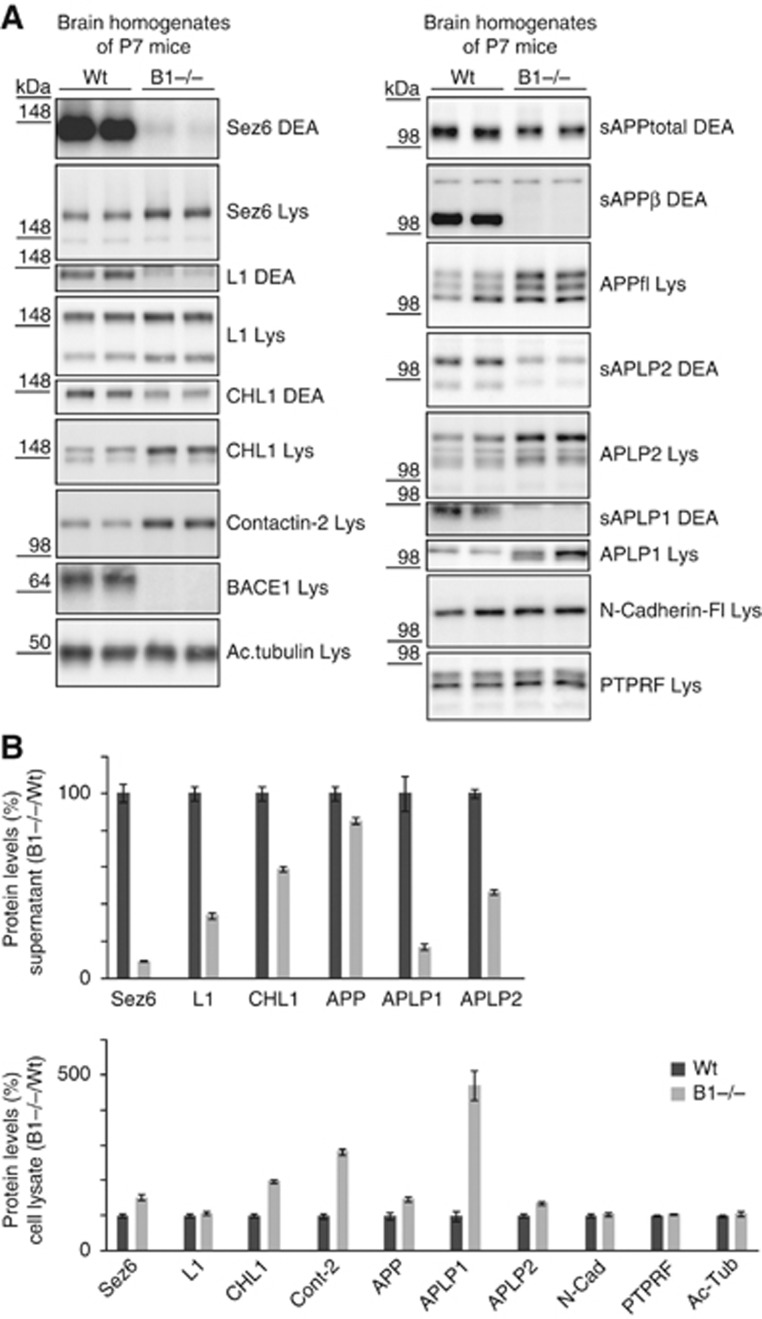
Figure 4.
Validation of BACE1 substrates in brain extracts of P7 wild-type and BACE1 knock-out mice. (A) P7 brains from wild-type (wt) and BACE1 (B1−/−) knock-out mice were separated into a soluble (DEA) and a membrane fraction (Lys), containing the soluble proteins (DEA) and the membrane proteins (Lys), respectively. The soluble ectodomains of SEZ6, CHL1 and L1 were reduced in the DEA fraction, while the full-length products had increased levels in the lysate. For contactin-2, the increased levels were observed in the membrane fraction. No change was observed in the soluble fraction, because the GPI-anchored membrane form of the protein was also solubilized with the prolonged DEA extraction in this experimental series. The BACE1-specfic cleavage product APPsβ and BACE1 expression in the lysate served as internal controls. Additionally, proteolytic processing of the APP family and of the two negative controls N-Cadherin and PTPRF was investigated. (B) Quantification of data from (A). The ratio of B1−/−/Wt for all four proteins in the DEA and the lysate fraction is shown. Quantification includes the positive controls APP, APLP1, APLP2 and the negative controls PTPRF and N-Cadherin, which are not BACE1 substrates. Given are mean and s.e.m. (n=6). Figure source data can be found with the Supplementary data.