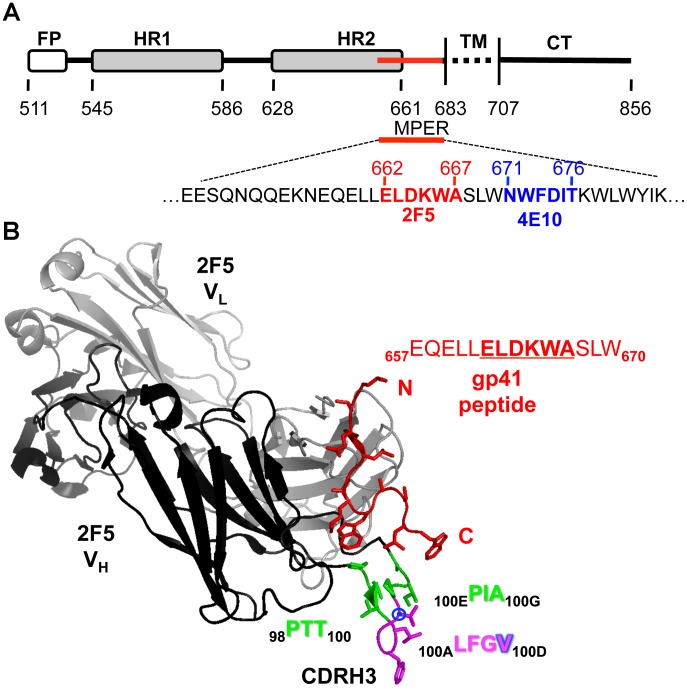
Figure 1. HIV-1 envelope glycoprotein gp41 schematic and structure of the 2F5 antibody-peptide complex.
(A) The 2F5 (red) and 4E10 (blue) contiguous epitopes lie within the membrane proximal external region (MPER) of the HIV transmembrane envelope glycoprotein, gp41. (B) Crystal structure (PDB: 1TJI) of the antibody 2F5 heavy chain (black) and light chain (gray) in complex with the gp41 peptide (red). The third complementarity-determining region of the 2F5 heavy chain (CDRH3) is colored in green and magenta. Residues colored green (98PTT100 and 100EPIA100G) were removed to decrease the length of the CDRH3 loop. Insertions were also engineered into this region to elongate the length of the loop. Residues at the apex of the CDRH3 in magenta (100ALFGV100D) were not altered. The residue V100D is marked by the blue circle as this residue is substituted for by a W in several of the 2F5 variant antibodies.