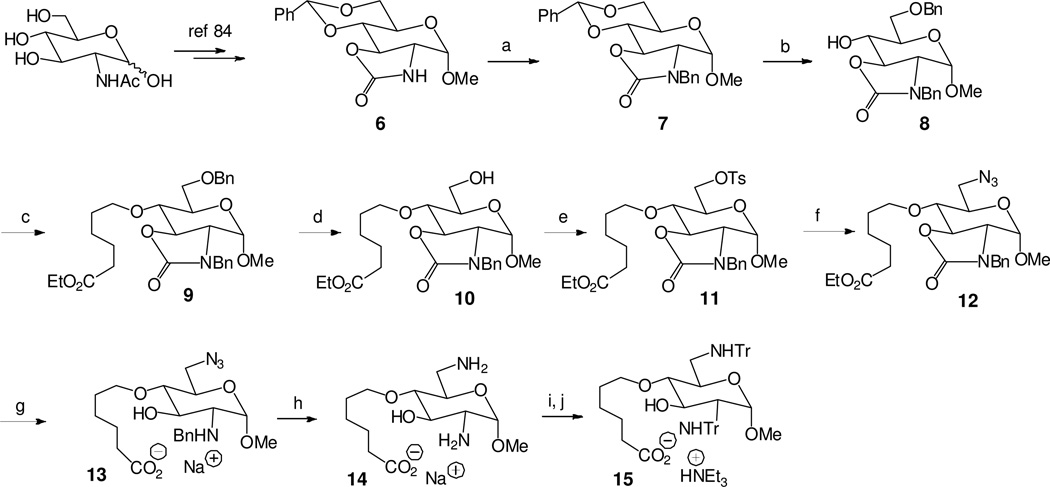
Scheme 1.
a Synthesis of the glucosamine derivative 15 for further coupling to the anti-TAR PNAs.
aReagents and conditions: (a) BnBr, K2CO3, DMF, rt, 10 h, 84%; (b) Et3SiH, BF3.OEt2, CH2Cl2, 0 °C, 2 h, 81%; (c) 6-bromohexanoic acid ethyl ester, NaH, DMF, rt, 8 h, 92%; (d) H2, Pd/C (10%), EtOH, rt, 12 h, 98%; (e) TsCl, pyridine; rt, 10 h, 96%; (f) NaN3, DMF, 80 °C, 3 h, 91%; (g) 1M NaOH, dioxane/H2O (4/1), 100 °C, 14 h, 74%; (h) HCO2NH4, Pd(OH)2/C (20%), MeOH/H2O (9/1), reflux, 1.5 h, 95%; (i) TrCl, DMF, Et3N, rt, 8 h, 68%; (j) 1M NaOH/dioxane (1/1), 80 °C, 6 h, 85%.