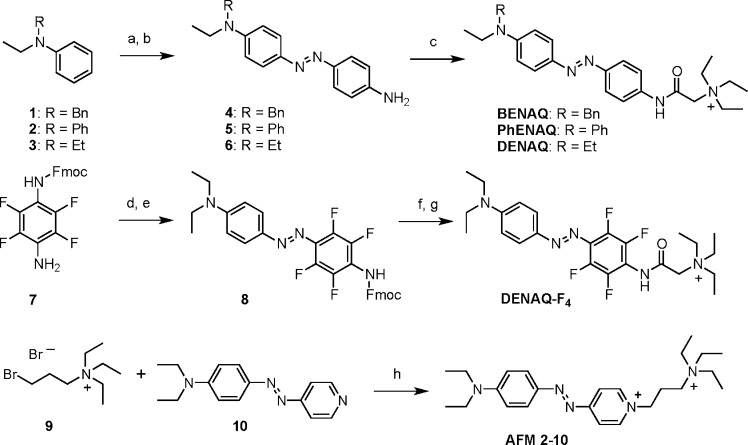
Scheme 1. Synthesis of BENAQ, PhENAQ, DENAQ, DENAQ-F4, and AFM 2-10.
Reagents and conditions: (a) 4-nitroaniline, isoamylnitrite, HCl, MeOH (87% for 1, 79% for 2, 62% for 3); (b) Na2S, H2O, 1,4-dioxane, 90 °C (88% for 4, 42% for 5, 88% for 6); (c) 2-triethylammonium acetic acid chloride, DIPEA, DMF, 0 °C to RT (67% for BENAQ, 66% for PhENAQ, 92% for DENAQ); (d) BF3 Et2O, isoamylnitrite, THF −40 to −5 °C; (e) diethylaniline, NaOAc, 0 °C to RT (36% over two steps); (f) piperidine, Et2O, 0 °C to RT (85%); (g) 2-triethylammonium acetic acid chloride, DIPEA, DMF, 0 °C to RT (38%); (h) DMF, 80 °C (92%).