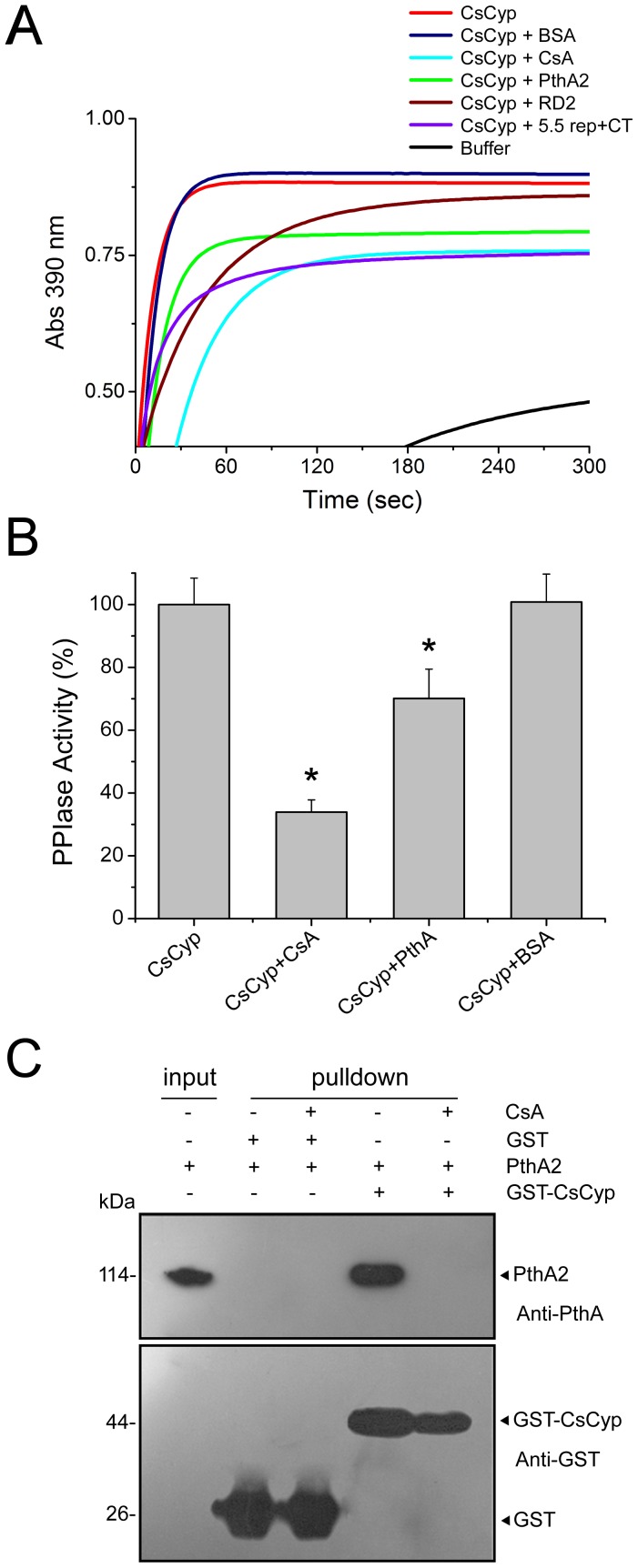
Figure 5. PthA2 inhibits the PPIase activity of CsCyp.
(A) The PPIase activity of recombinant CsCyp protein was evaluated by the α-chymotrypsin-coupled assay. Enzyme activity was measured immediately after the addition of α-chymotrypsin and the substrate into the reaction mixture. The PPIase activity of CsCyp reached a plateau within 30 to 60 s after the start of the reaction (red). This activity was drastically reduced in the presence of 30 nM CsA (light blue), as compared to buffer only, as control (black). PthA2 (green), its internal repetitive DNA-binding domain RD2 (brown), or its C-terminal domain+5.5 internal repeats (purple) but not BSA (dark blue), added into the reaction mixture reduced the PPIase activity of CsCyp in a time-course measurement. (B) PPIase activity of CsCyp in the absence and presence of CsA (30 nM), PthA2 or BSA (15 nM). Activities were the mean of three independent measurements recorded 30 s after the start of the reaction, and the asterisks indicate statistically different means relative to that of normal activity. (C) GST-pulldown assay using the GST-CsCyp as bait and purified PthA2 as prey. Protein samples were electrophoresed and probed with the anti-GST and anti-PthA sera. PthA2 bound to the GST-CsCyp only and the cyclophilin inhibitor CsA abrogated the interaction. Purified PthA2 used as input and the molecular sizes of the corresponding proteins are shown on the left.