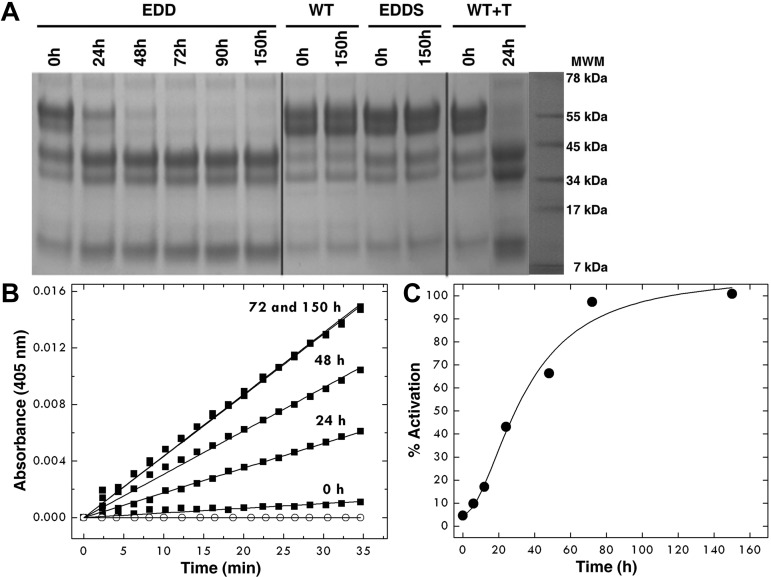
Figure 1.
Autoactivation of the EDD mutant of protein C. After affinity chromatography, the protein concentration was adjusted to 0.8 mg/mL and protein C was left at room temperature for 150 hours. (A) SDS-PAGE electrophoresis documents the spontaneous conversion of the EDD mutant of protein C to activated protein C with disappearance of the band at 55 kDa and thickening of the bands in the 34- to 45-kDa range. The chemical identity of the downshifted bands as the activated product was confirmed by N-terminal sequencing and mapped to the sequence LIDGK corresponding to the new N-terminus of the heavy chain of activated protein C. Double bands reflect the intrinsic heterogeneity of protein C resulting from posttranslational modifications.39 The profile after 72 hours becomes identical to that observed on activation of wild-type protein C with thrombin. No autoactivation is observed with wild-type protein C and the inactive mutant EDDS up to 150 hours. (B) The kinetics of autoactivation was monitored from hydrolysis of DRR, a chromogenic substrate specific for activated protein C, under experimental conditions of 5mM Tris, pH 7.4, 145mM NaCl, 2mM CaCl2, 0.1% PEG8000 at 37°C. ○ represents wild-type protein C. (C) The amount of activation of the protein C mutant EDD was monitored over time from the progress curves in panel B and converted into a percentage. The sigmoidal shape of the curve is indicative of the presence of intermediates along the autoactivation pathway, consistent with an autocatalytic process.