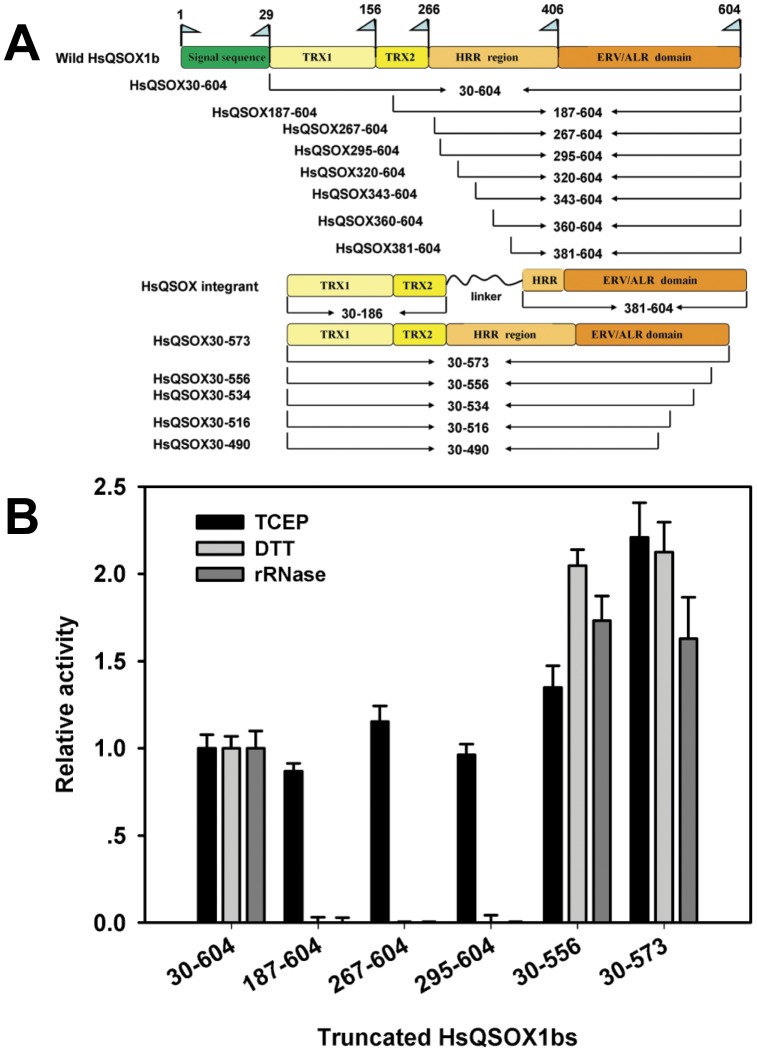
Figure 1. Schematic diagram of HsQSOX1b truncation and activity of the partial truncated variants.
(A) A series of truncated enzymes were prepared according to the nine QHZs. Their secondary structures were verified through crystallization and prediction, and their names were formulated according to their amino acid boundaries. (B) The oxidase activities of HsQSOX1b truncated variants toward TCEP, DTT and rRNase, relative to those of the wildtype enzyme, respectively. The oxidase activity was based on determining the rate of the H2O2 generation [22]. The N-terminal truncated variants, namely, HsQSOX1b187–604, HsQSOX1b267–604, and HsQSOX1b295–604, retained their oxidase activity to TCEP but lost their thiol oxidase activity to reduced RNase and DTT. HsQSOX1b30–573 and HsQSOX1b30–556 had higher thiol oxidase activity than the full-length enzyme, and both the thiol and TCEP oxidase activities of HsQSOX1b30–573 were higher than those of HsQSOX1b30–604 and HsQSOX1b30–556.