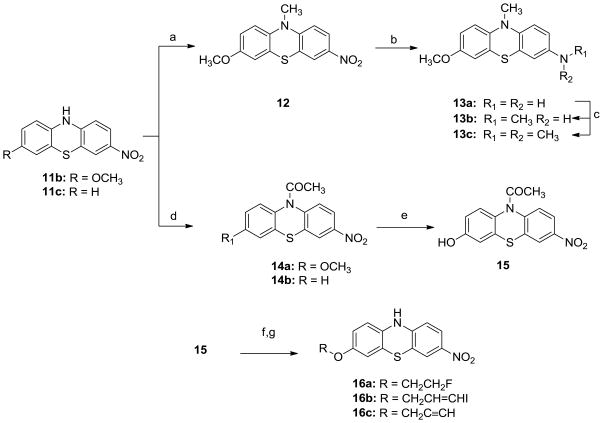
Scheme 3.
Synthesis of N-substituted analogues 12, 13a–c, 14a–b, 15, 16a–c.
Reagents and conditions:
a) NaH, CH3l, DMF, 0°C, overnight; b) H2, Pd-C (10%), 90 psi, ambient temperature, overnight, c) CH3l, Na2CO3, MeCN, ambient temperature, overnight; d) CH3COCl, DCM, ambient temperature, overnight; e) BBr3/DCM (1M), DCM, −78°C, overnight. f) 1-bromo-2-fluroethane, 3-bromopropyne or 3-bromo-1-iodopropene, Na2CO3, MeCN; g) HCl (3 M), H2O, reflux, 5h;