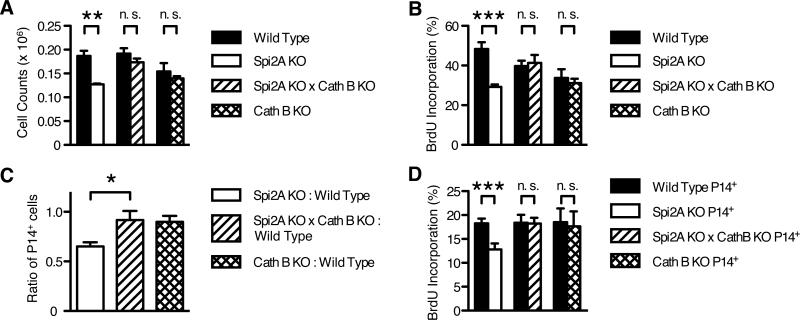
Figure 7. Concomitant cathepsin B deficiency restores the reduced number and homeostatic proliferation of Spi2A-deficient memory-phenotype CD44hi CD8+ and memory CD8+ T cells.
(A-B) 15-week-old mice were dissected and stained as in Figure 1. (A) Number of CD44hi CD8+ T cells present in one femur. (B) BrdU incorporation of CD44hi CD8+ T cells after one week of BrdU administration. Significance values were calculated by two-way ANOVA with Bonferroni post-test (**, P < 0.01; ***, P < 0.001). (C-D) CD8+ splenocytes from gp33 epitope-specific P14 TCR transgenic wild type (CD45.1+) mice and either Spi2A KO, Spi2A KO × Cath B KO, or Cath B KO (CD45.2+) mice were isolated and mixed in even ratios. 5000 P14+ CD8+ cells were adoptively transferred into CD45.1+2+ hybrid congenic recipients, which were subsequently infected with LCMV, as described in Figure 3. (C) Ratio of Spi2A KO, Spi2A × Cath B KO, or Cath B KO : Wild Type among donor P14+ CD8+ cells in the spleen 70-76 days post infection. Significance values were calculated by one-way ANOVA with Bonferroni post-test (*, P < 0.05). (D) BrdU incorporation of P14+ CD8+ cells in spleen and bone marrow after one week of BrdU administration in mice 70-75 days post infection. Significance values were calculated by paired Student's two-tailed t test (***, P < 0.001). (A-D) Data show mean ± SEM of at least n = 5 mice and are representative of at least 2 independent experiments.