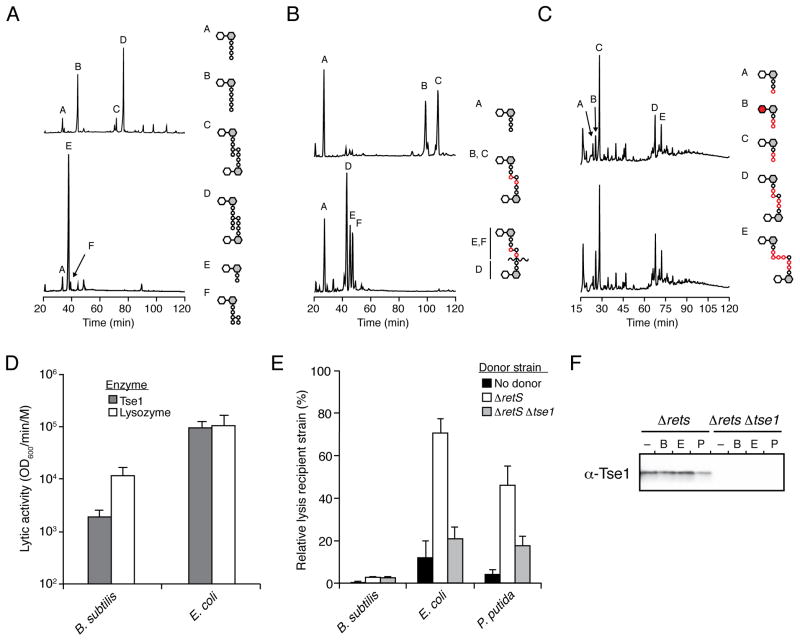
Figure 4. Tse1 is specific for Glu-mDAP-type peptidoglycan.
(A–C) Tse1 degrades only Glu-mDAP-type peptidoglycan of E. coli and B. subtilis and not the Lys-type peptidoglycan of S. pneumoniae. Partial HPLC chromatograms of sodium borohydride-reduced soluble E. coli (A), B. subtilis (B) or S. pneumoniae(C) peptidoglycan sacculi products resulting from digestion with cellosyl (top) or with Tse1 followed by cellosyl (bottom). Assignments of Tse1 cleavage products were made based on MS analysis (Panel A, Peaks E and F; Figure S3A) or prior work (Bui et al., 2012; Russell et al., 2011). Structures corresponding to the major peaks are shown schematically with hexagons and circles representing sugars and amino acid residues, respectively. Reduced sugar moieties are shown with grey fill. Modifications or substitutions relative to typical Gram-negative peptidoglycan are indicated with red fill.
(D) Tse1 can lyse B. subtilis cells in vitro. Activity of purified Tse1 and lysozyme against B. subtilis and EDTA-permeabilized E. coli. Error bars +/− s.d. n=3. See also Figure S3C.
(E) Tse1 delivered by the H1-T6SS does not contribute to lysis of B. subtilis. Relative lysis as measured by supernatant LacZ activity of the indicated recipient organisms in growth competition under T6SS-promoting conditions with the indicated P. aeruginosa strains. Error bars +/− s.d. n=3.
(F) Tse1 is secreted by Δrets P. aeruginosa. Western blot analysis of total extracellular Tse1 levels in competition assays against B. subtilis, E. coli, and P. putida.