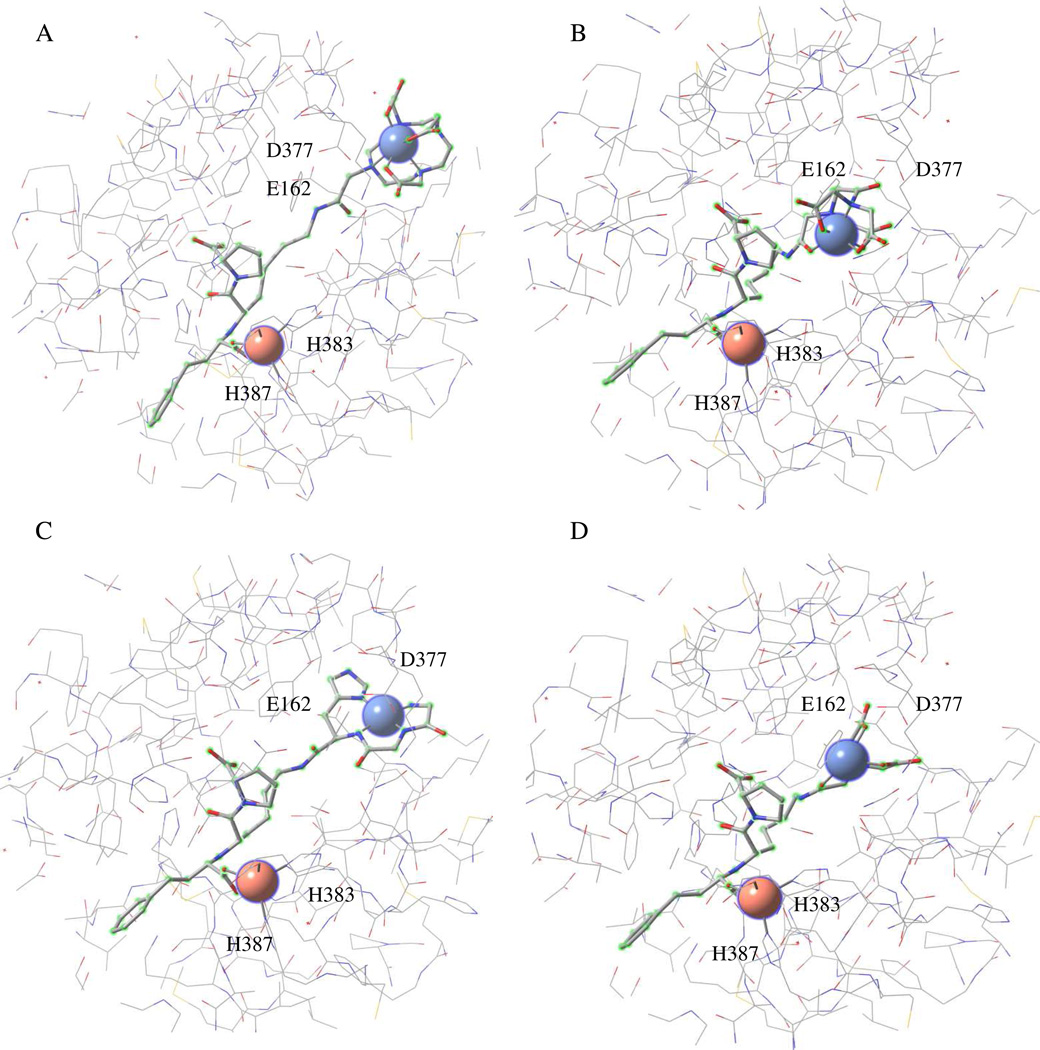
Figure 3.
Energy-minimized structural models of tACE active site (equivalent to the active site of C-domain of sACE except for 36 additional residues at the N-terminus of tACE). The models show binding by (A) Fe-DOTA-lisinopril, (B) Fe-EDTA-lisinopril, (C) Cu-GGH-lisinopril, and (D) Fe-NTAlisinopril. The redox-active metal for each metal-chelate-lisinopril complex is shown as a blue sphere (upper right within each model), and the active site Zn2+ is shown as a pink sphere (bottom left within each model). Residues E162 and D377 can interact with the lysine sidechain of unmodified lisinopril, while H383 and H387 form part of the conserved HEXXH Zn2+-binding motif.