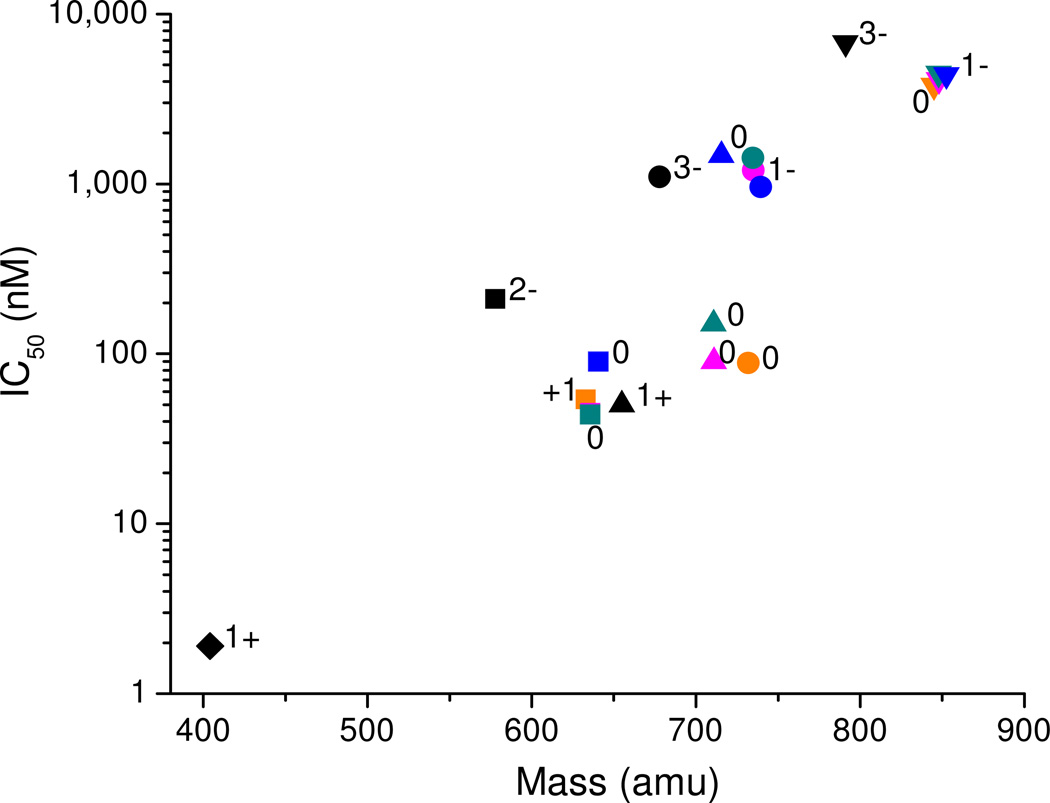
Figure 6.
sACE-1 binding-affinity of M-chelate-lisinopril complexes was inversely correlated with the size and negative charge of the species attached to the lysine sidechain of lisinopril. Lisinopril and all M-chelate-lisinopril species (metal-bound and metal-free) are shown: ♦ = lisinopril; ■ = NTA-lisinopril; ▲ = GGH-lisinopril; ● = EDTA-lisinopril; ▼ = DOTA-lisinopril. Orange = Fe; pink = Co; cyan = Ni; blue = Cu; black = no metal. The charge of the modified lysine sidechain of lisinopril is listed for each attachment; the charge for each Fe3+ complex was 1+ higher than for each corresponding M2+ complex.