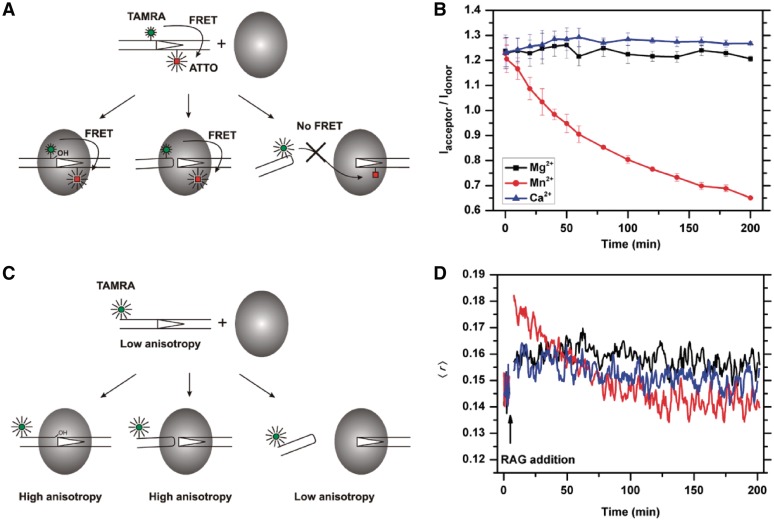
Figure 1.
Real-time analysis of RAG-RSS interactions using fluorescence-based techniques. (A). Schematic representation of the FRET changes that result from RAG-mediated interactions with the TAMRA-ATTO doubly labeled 12RSS. The FRET signal remains unchanged during nicking (left) and hairpin formation (middle), as long as the HP-CEs are kept within the coding end complex (CEC) at close proximity with signal ends (SEs). The release of the HP-CEs from the CEC (right) results in an increase in donor (TAMRA), and a reduction in acceptor (ATTO), producing a reduction in FRET efficiency. (B) c/cRAG mediated single cleavage reactions in the presence of different cations revealed by FRET. The ratio of acceptor to donor (Iacceptor/Idonor) is compared among the cleavage reactions in the presence of Ca2+ (blue), which does not support cleavage, Mg2+ (black), which causes nicking only, or Mn2+ (red), which allows the uncoupled cleavage. The results are presented as an average of three replicates with the SD. (C) Schematic illustration of the expected changes in fluorescence anisotropy due to interactions between RAG and the 5′-TAMRA labeled 12RSS. The anisotropy signal increases once the RAG protein binds to the 12RSS. The elevated anisotropy signals are maintained after RAG-mediated nicking or excision, as long as the HP-CEs remain in the CEC. The release of HP-CEs from the CEC results in the reduction of the anisotropy signal. (D) Cation-dependent changes in fluorescence anisotropy during RAG-RSS interactions. The anisotropy profiles of c/cRAG interactions with the TAMRA-labeled 12RSS are affected by the cation present in the cleavage reaction, i.e. Ca2+ (blue), Mg2+ (black) or Mn2+ (red).