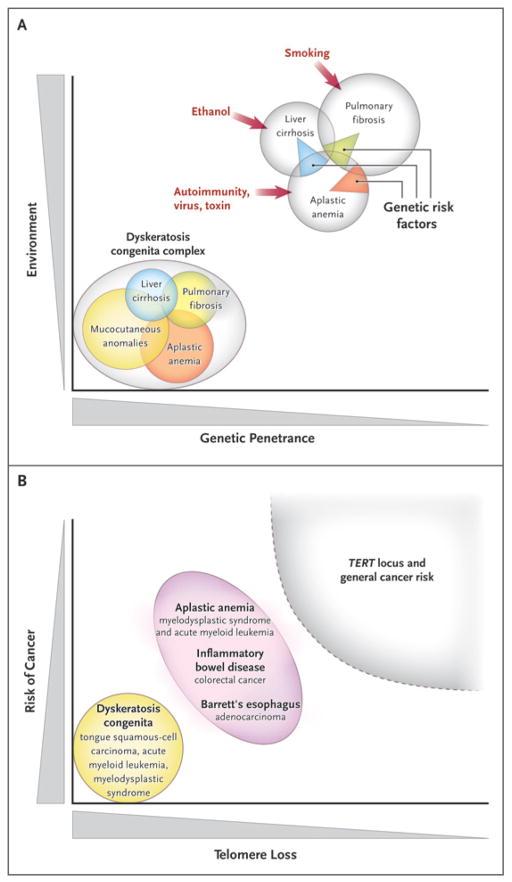
Figure 5. Telomere Erosion and Human Diseases.
Panel A shows a Venn diagram of mutations of the telomerase complex and human telomere diseases. Dyskeratosis congenita is the most evident and severe manifestation of genetic lesions causing telomere diseases, with high genetic penetrance and congenital clinical manifestations. However, telomerase mutations may be less penetrant and induce single-organ damage in adults without suggestive family histories and the classic physical signs of dyskeratosis congenita. Thus, telomerase mutations represent risk factors rather than genetic determinants in aplastic anemia, pulmonary fibrosis, and liver cirrhosis. Environmental, epigenetic, and other genetic factors probably contribute to disease development in these patients. Panel B shows the relationship between telomere shortening and the risk of cancer. In dyskeratosis congenita, in which genetic penetrance is high, the risk of the development of cancer — particularly head and neck squamous-cell carcinoma and acute myeloid leukemia — also is elevated. In addition, patients with aplastic anemia are at risk for the development of clonal malignant disorders, but the risk is lower than that among patients with dyskeratosis congenita. Similarly, short telomeres appear to predict the progression of chronic inflammatory gastrointestinal states to adenocarcinoma. In multiple genomewide association studies, the TERT locus has appeared as a significant susceptibility locus for a variety of cancers, but at relatively low odds ratios. Shaded areas representing diseases and disease states are not drawn to scale.