Sir,
Melasma (from the Greek word, “melas” meaning black) is a common, acquired, circumscribed hypermelanosis of sun-exposed skin. It presents as symmetric, hyperpigmented macules having irregular, serrated, and geographic borders. The most common locations are the cheeks, upper lips, the chin, and the forehead, but other sun-exposed areas may also occasionally be involved.[1] Melasma occurs in all skin types and in people of all racial and ethnic groups, but is more common in those with darker complexions living in areas of intense UV radiation, such as Latin Americans, Asians, and Blacks.[2] It is the most common pigmentary disorder among Indians.[3]
Based on Wood's light findings, Sanchez et al.[4] classified melasma into four subtypes: epidermal, dermal, mixed, and Wood's light inapparent. Wood's light may also serve as a prognostic guide in the treatment of melasma, as the epidermal type of melasma is more likely to respond favorably to depigmenting agents than other types. Wood's lamp can be used to determine the depth of melanin in the skin. The variations in epidermal pigmentation become more apparent under Wood's light. For dermal pigmentation, this contrast is less pronounced. However, this applies only for the fair skin types and not for type V or VI skin.[5] Three clinical patterns of distribution of the pigmentation may be recognized: Centro facial, malar, and mandibular.
In India, over-the-counter medications are often tried by patients before approaching a dermatologist. In 2004, a triple combination of hydroquinone (2%) with tretinoin (0.025%) and mometasone furoate (0.1%) was launched in India. This combination has a potent steroid mometasone furoate (0.1%) which induces local side effects like thinning of skin, persistent erythema, and telangiectasia within few weeks of application. Patient keeps on applying this combination as it can be purchased without prescription in India.
We studied the various agents used by patients before approaching a dermatologist. Fifty consecutive patients of melasma in the age group of 18-45 years with a mean age of 25.5 years were included in this study. There were 38 females and 12 males. The duration of melasma ranged from 1 to 6 years with a mean of 1.1 years.
We found the following medications were used by patients before approaching a doctor. Most were advised these medications by friends, parents, and beauticians [Table 1].
Table 1.
Use of medications by patients

Most patients who used mometasone-based triple creams found clearing of melasma followed by relapse. Red skin after use of triple creams was noted by 15 out of 23 patients. We found only two patients who used sunscreens with triple creams.
Clinical examination revealed telangiectasia (8 out of 23) and skin thinning (4 out of 23) as side effects of mometasone-based creams [Figure 1].
Figure 1.
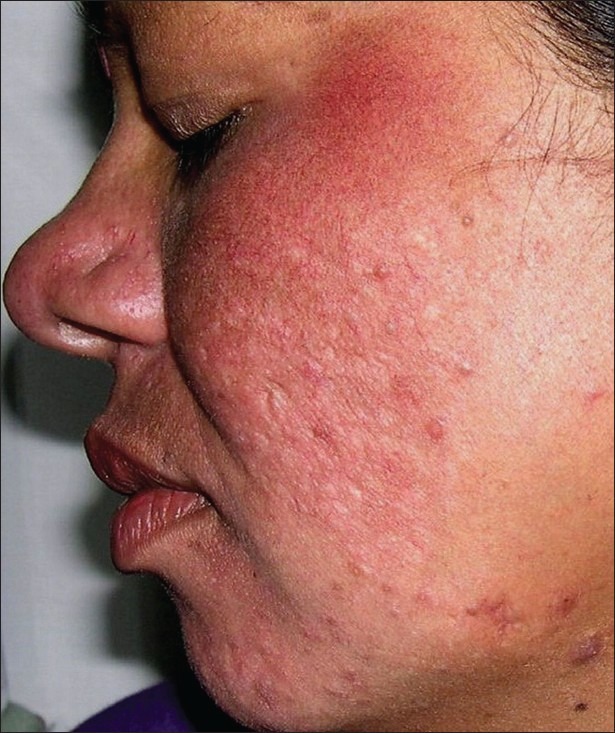
Persistent erythema after use of mometasone-based triple cream
Majid found that majority of patients (51.7%) had used the mometasone-based triple combination treatment well beyond the recommended duration. Steroid side effects were seen in 26 patients (43.3%).[6] The recommendation that the combination be stopped after about 4–8 weeks is followed rarely at all by the patients. Easy availability of these creams over-the-counter at a pocket-friendly price makes the patients to use this combination for a long duration. Prolonged use makes facial skin sun sensitive. Patients cannot use routine cosmetics on sensitive skin. Rubbing triple creams on hyperpigmented area vigorously worsens the situation in most patients. We found 2 patients out of 10 (20%) who used triple combination with mometasone and glycolic acid peels, with side effects of persistent erythema, irritation, and hyperpigmentation.[7] Now, triple combination cream with fluocinolone acetonide (0.01%) as steroid is available in India. Fluocinolone-based triple creams are safe for long-term use up to 24 weeks. Risk of skin atrophy with 24-week use of triple combination cream with fluocinolone for the treatment of melasma is very low.[8] We found this fluocinolone-based triple combination along with glycolic acid peels as a safe and effective option in Indian patients.[9]
We suggest triple combination creams with mometasone should be prescribed after thorough counseling with the patient. Long-term side effects of this cream should be explained to the patient.
References
- 1.Bandyopadhyay D. Topical treatment of melasma. Indian J Dermatol. 2009;54:303–9. doi: 10.4103/0019-5154.57602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor S. Epidemiology of skin diseases in ethnic populations. Dermatol Clin. 2003;21:6017. doi: 10.1016/s0733-8635(03)00075-5. [DOI] [PubMed] [Google Scholar]
- 3.Pasricha JS, Khaitan BK, Dash S. Pigmentary disorders in India. Dermatol Clin. 2007;25:343–522. doi: 10.1016/j.det.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez NP, Pathak MA, Sato S. Melasma: A clinical, light microscopic, ultrastructural, and immunofluorescence study. J Am Acad Dermatol. 1981;4:698–710. doi: 10.1016/s0190-9622(81)70071-9. [DOI] [PubMed] [Google Scholar]
- 5.Gilchrest BA, Fitzpatrick TB, Anderson RR, Parrish JA. Localization of melanin pigmentation in the skin with Wood's lamp. Br J Dermatol. 1977;96:245–8. doi: 10.1111/j.1365-2133.1977.tb06132.x. [DOI] [PubMed] [Google Scholar]
- 6.Majid I. Mometasone-based triple combination therapy in melasma: Is it really safe? Indian J Dermatol. 2010;55:359–62. doi: 10.4103/0019-5154.74545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godse KV. Triple combination of hydroquinone, tretinoin and mometasone furoate with glycolic acid peels in melasma. Indian J Dermatol. 2009;54:92–3. doi: 10.4103/0019-5154.49005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhawan J, Grimes P, Pandya AG, Keady M, Byers HR, Guevara IL, et al. A histological examination for skin atrophy after 6 months of treatment with fluocinolone acetonide 0.01%, hydroquinone 4%, and tretinoin 0.05% cream. Am J Dermatopathol. 2009;31:794–8. doi: 10.1097/DAD.0b013e3181a9070d. [DOI] [PubMed] [Google Scholar]
- 9.Godse KV, Sakhia J. Triple combination and glycolic acid peels in melasma in Indian patients. J Cosmet Dermatol. 2011;10:68–9. doi: 10.1111/j.1473-2165.2010.00540.x. [DOI] [PubMed] [Google Scholar]


