Abstract
AIM
To investigate and characterise an inborn error of metabolism in a dog with skeletal and ocular abnormalities.
METHODS
A 2.5-year-old small male Miniature Poodle-like dog was presented with gross joint laxity and bilateral corneal opacities. Clinical examination was augmented by routine haematology, serum chemistry, radiographs, pathology, enzymology and molecular genetic studies. Euthanasia was requested when the dog was 3 years of age because of progressively decreasing quality of life.
RESULTS
Radiology revealed generalised epiphyseal dysplasia, malformed vertebral bodies, luxation/subluxation of appendicular and lumbosacral joints with hypoplasia of the odontoid process and hyoid apparatus. These clinical and radiographic findings, together with a positive urinary Berry spot test for mucopolysaccharides, and metachromatic granules in leucocytes, were indicative of a mucopolysaccharidosis (MPS), a lysosomal storage disease. Histological lesions included vacuolation of stromal cells of the cornea, fibroblasts, chondrocytes, macrophages and renal cells. The brain was essentially normal except for moderate secondary Wallerian-type degeneration in motor and sensory tracts of the hind brain. Dermatan sulphateuria was present and enzymology revealed negligible activity of N-acetylgalactosamine-4-sulphatase, also known as arylsulphatase B, in cultured fibroblasts and liver tissue. A novel homozygous 22 base pair (bp) deletion in exon 1 of this enzyme’s gene was identified (c.103_124del), which caused a frame-shift and subsequent premature stop codon. The “Wisdom pure breed-mixed breed” test reported the dog as a cross between a Miniature and Toy Poodle.
CONCLUSIONS
The clinicopathological features are similar to those of MPS type VI as previously described in dogs, cats and other species, and this clinical diagnosis was confirmed by enzymology and molecular genetic studies. This is an autosomal recessively inherited lysosomal storage disease.
CLINICAL RELEVANCE
The prevalence of MPS VI in Miniature or Toy Poodles in New Zealand and elsewhere is currently unknown. Due to the congenital nature of the disorder, malformed pups may be subject to euthanasia without investigation and the potential genetic problem in the breed may not be fully recognised. The establishment of a molecular genetic test now permits screening for this mutation as a basis to an informed breeding policy.
Keywords: Lysosomal storage disease, mucopolysaccharidosis, Arylsulphatase B, Miniature/Toy Poodle, mutation
Introduction
Mucopolysaccharides are complex sulphated sugars with repeating disaccharide motifs that form sulphated glycosaminoglycans (GAG). There are five different GAG: chondroitin 4-sulphate, chondroitin 6-sulphate, heparan sulphate, dermatan sulphate and keratan sulphate. In their synthesis, each GAG is attached to a specific protein core to produce a variety of proteoglycans with many different biological functions. The mucopolysaccharidoses are a group of lysosomal storage diseases caused by deficient activity of enzymes associated with the lysosomal degradation of one, or sometimes several GAG (Neufeld and Meuezner 2001; Haskins and Giger 2008). The clinical signs and pathological lesions are related to the distribution and functions of the GAG involved and as such, are indicative of the specific enzyme defect.
Lysosomal storage disorders of animals are important as they affect health and cause loss of production (Jolly and Walkley 1997). They can, and should be controlled on economic and welfare grounds by use of specific biochemical and/or molecular tests. They are also very important as animal models of human lysosomal storage diseases, originally to help understand their pathogenesis, but more recently as models for specific therapeutic experiments (Crawley et al. 1997; Auclair et al. 2003; Haskins et al. 2006). This case report describes mucopolysaccharidosis (MPS) type VI in a Miniature Poodle-type dog.
Materials and methods
History and clinical presentation
A small deformed male Miniature Poodle-like dog was nursed by the owner since puppyhood. A family history was not available. When presented for clinical examination at 2.5 years of age, the dog was bright and alert, but appeared stunted (2.25 kg bodyweight), could stand only with assistance and moved about with difficulty. There was marked reduction in muscle mass and severe laxity of joints with crepitus of hip, stifle, elbow and carpal joints. The dog had severe dental calculi and the tongue appeared slightly enlarged with soft fleshy bilateral outgrowths of tissue. Submaxillary salivary glands and retropharyngeal lymph nodes were enlarged. Both eyes exhibited moderate corneal opacities (Figure 1). The liver was palpable beyond the costal arch and a left-sided soft systolic murmur was auscultated. While the owner raised and maintained the dog with a high standard of nursing care, euthanasia was requested at 3 years of age because of progressively decreasing quality of life.
Figure 1.
Corneal opacity in a Miniature Poodle-like dog aged 2.5-year-old, diagnosed with mucopolysaccharidosis type VI.
Clinical investigation
The affected dog was evaluated by physical examination. Blood was collected by jugular venipuncture and routine haematology and biochemistry were performed at commercial laboratories (Gribbles Veterinary Pathology, Palmerston North, New Zealand and New Zealand Veterinary Pathology Ltd., Palmerston North, New Zealand). Fresh blood smears were stained separately with Leishman (Fisher Scientific, UK Ltd., Loughborough, UK) and Diff-Quick (Siemans Healthcare Diagnostics Inc., Newark NJ, USA). Urine was collected via cystocentesis and assessed using a modified Berry MPS spot test for the presence of GAG (Chih-Kuang et al. 2002). Urinary GAG were then separated by high-resolution electrophoresis (Hopwood and Harrison 1982).
Pathological investigation
Following euthanasia by intravenous phenobarbitone, whole body radiography and necropsy were performed. Tissues were fixed in 10% buffered formalin and later processed into paraffin wax and epoxy resin. Paraffin sections were stained with H&E and epoxy resin sections were stained with 2% Toluidine blue. Impression smears of liver and spleen were stained with Leishman stain.
Enzymology and molecular genetic studies
Fibroblasts were cultured from skin of the affected dog as well as from two normal control dogs as described for primary human skin fibroblast culture (Rittie and Fisher 2005). Urine and liver specimens were freshly frozen for biochemical studies. Liver and cultured skin fibroblasts from the affected dog and two control dogs were assayed for N-acetylgalactosamine-4-sulphatase, also known as arylsulfatase B (E.C.3.1.6.1) by the method of Hein et al. (2005), and N-sulphoglucosamine sulphohydrolase (sulphamidase, E.C.3.10.1.1) activity by the method of Hopwood and Elliott (1982).
Genomic DNA was extracted from frozen blood using the Qiagen DNeasy Blood and Tissue Kit (Qiagen Inc., CA, USA) and examined for known mutations in miniature pinschers and miniature schnauzers (PennGen, College of Veterinary Medicine, University of Pennsylvania, Philadelphia, USA). Exons from the arylsulphatase B gene were amplified, run on 6% polyacrylamide gels and directly sequenced according to standard molecular genetics techniques. For amplification, an initial denaturation step at 95°C for 5 minutes was followed by 35 cycles of denaturation at 95°C for 1 minute, annealing at 62°C for 1 minute, extension at 72°C for 3 minutes, and a final extension step at 72°C for 10 minutes with a Taq polymerase mixture for GC-rich products. An amplicon from exon 1 (predicted size 363 base pair (bp)) was identified on a 6% polyacrylamide gel after electrophoresis and then directly sequenced and compared with that of canine arylsulphatase B (NM_001048133.1). Genomic DNA was also assessed with the commercially available “Wisdom pure breed-mixed breed” test to determine the breed composition (www.wisdompanel.com).
Results
Clinical pathology, cytology and urinalysis
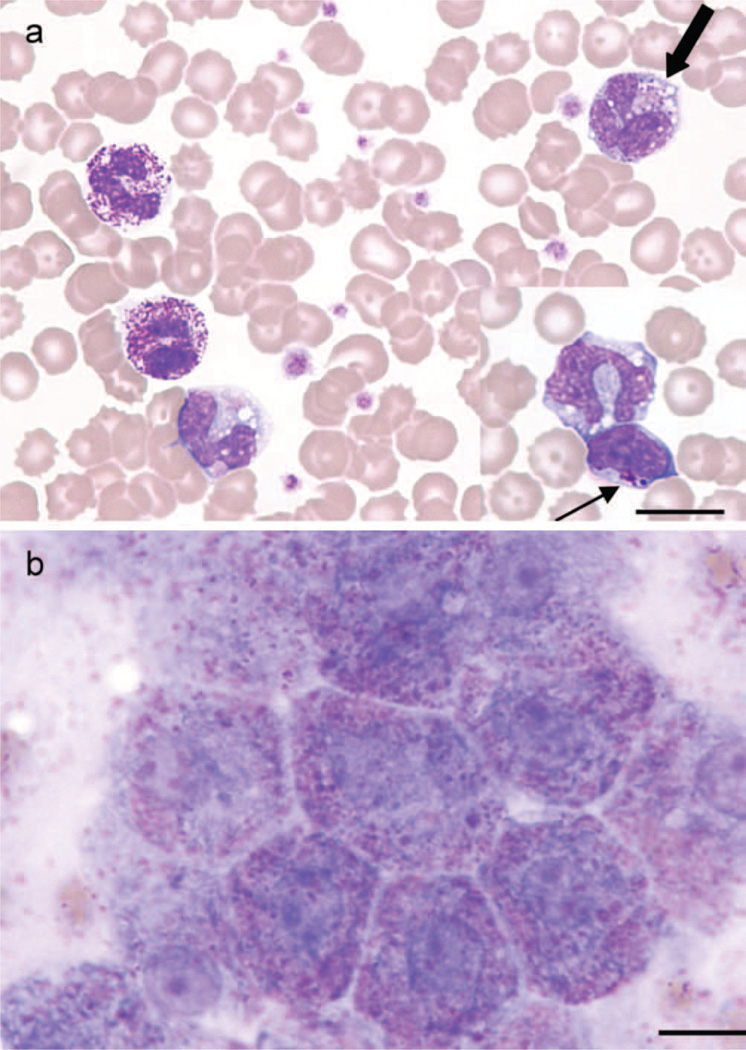
Haematology revealed a mild regenerative anaemia and a leucocytosis due to a mild neutrophilia and monocytosis. In Leishman stained blood smears, many coarse metachromatic intracytoplasmic inclusions (Alder-Reilly bodies) were noted mostly in neutrophils, but also in lymphocytes and monocytes (Figure 2a). Similar inclusions were seen in hepatocytes in an impression smear taken at necropsy (Figure 2b). Of particular note is that the inclusions were not obvious in Diff-Quick-stained smears. Serum biochemistry results showed only mild non-specific changes.
Figure 2.
(a) Blood smear from a Miniature Poodle-like dog diagnosed with mucopolysaccharidosis type VI showing metachromatic Alder-Reilly-type inclusions in neutrophils and macrophage (thick arrow), with similar inclusions in lymphocytes (inset, thin arrow) (Leishman stain, bar = 12 µm). (b) Impression smear of liver from the same dog taken at time of necropsy, showing pink-purple inclusions in hepatocytes (Leishman stain, bar = 10 µm).
Urine was hypersthenuric with 2+ protein. The modified Berry MPS spot test was positive showing mild metachromasia relative to a control urine. Subsequent high-resolution electrophoresis of urine demonstrated elevation of dermatan sulphate, without an increase of heparan sulphate.
Radiology
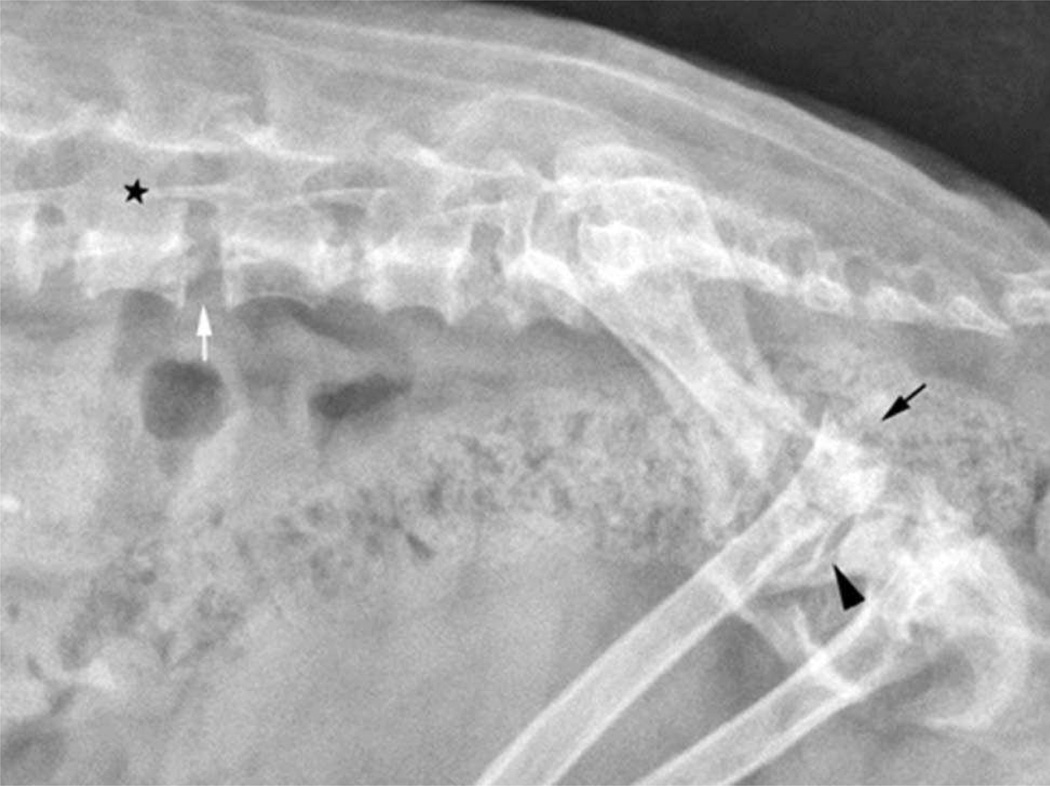
The vertebral bodies were shortened with an increase in vertebral canal diameter and their end plates were incompletely ossified resulting in widened and irregular margination of the intervertebral disc spaces (Figure 3). The odontoid process of the second vertebra (axis) had a blunted hypoplastic appearance. There were also shortened hyoid bones with widened synchondroses, a soft tissue swelling in the laryngeal region, and obliterated pharyngeal air columns.
Figure 3.
Lateral radiograph of the lumbosacral spine and pelvis of a Miniature Poodle-like dog, diagnosed with mucopolysaccharidosis type VI showing a reduction in length of the vertebral bodies with incomplete ossification of the end plates. The intervertebral disc spaces (white arrow) and vertebral canal (star) appear widened. There is severe subluxation of the sacrum with respect to L7 and dorsal luxation of one of the femoral heads (black arrow). The contralateral coxofemoral joint space is markedly widened (arrowhead).
Throughout the appendicular skeleton the epiphyses had a deformed appearance with cystic subchondral radiolucencies and incongruity of articular surfaces. There were varying degrees of subluxation to luxation of all the joints imaged including complete luxation of one coxofemoral joint and subluxation of the contralateral joint (Figure 3), bilateral elbow joint luxation, and subluxation of the intertarsal and tarsometatarsal joints. In the digits there was flaring of the distal metaphyseal regions of the metatarsal and metacarpal bones with a reduction in radiopacity of the epiphyses.
Pathology
The main gross physical abnormalities involving the skeleton, including a severe general laxity and luxation of joints, were as described clinically and radiologically. The bones were of normal hardness. There were bilateral corneal opacities and enlargement of the heart with severe thickening of bicuspid, tricuspid, and to lesser extent, pulmonary and aortic valves. In addition there was enlargement of the submaxillary salivary glands and retropharyngeal lymph nodes.
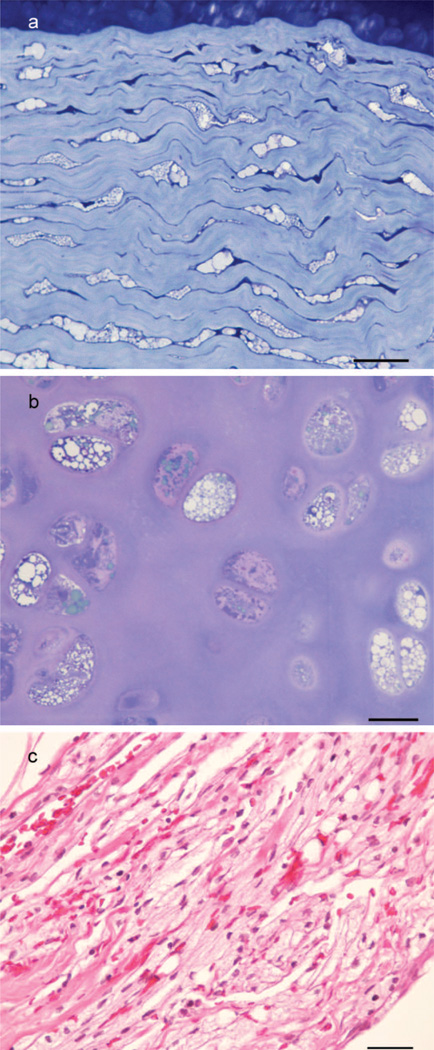
Histological abnormalities centred mainly on connective tissue lesions with vacuolation of fibroblasts including the stromal cells of the cornea (Figure 4a), and chondrocytes (Figure 4b). Accumulation of large numbers of macrophages occurred within myxomatous degenerative areas of heart valves, in lacunae within rib cartilage, in the medulla of lymph nodes, and to a lesser extent in the spleen, the interstitium of heart muscle, smooth muscle of intestines, pancreas, and submucosa of the bladder. In the liver these aggregates occurred around central veins and in hepatic triads, but Kupffer cells were also vacuolated. In the submaxillary salivary gland there was degeneration of acini and leakage of contents into the connective tissue which was infiltrated with large numbers of vacuolated macrophages. Vacuolation of epithelial cells of the kidney occurred, but was confined mainly to the proximal straight tubules of the loop of Henle.
Figure 4.
(a) Section of the cornea of a Miniature Poodle-like dog aged 3-year-old, diagnosed with mucopolysaccharidosis type VI showing vacuolated stromal cells (Epoxy resin, Toluidine blue, bar = 25 µm). (b) Section of tracheal cartilage from the same dog showing vacuolated chondrocytes (Epoxy resin, Toluidine blue, bar = 20 µm). (c) Section of leptomeninges from the same dog at the level of the caudal medulla showing cells infiltrated with highly vacuolated macrophages (Paraffin, H&E, bar = 25 µm).
The brain appeared normal except for moderate Wallerian-type degeneration of white matter in the pyramids of the posterior medulla extending into the ventral white matter of proximal cervical cord. In the dorso-lateral tracts of proximal cervical spinal cord, similar degeneration of white matter could be followed into the caudal cerebellar peduncles. In the caudal medulla there was long-standing Wallerian degeneration of the caudal bulbar nerve roots and of the ventral rootlet at C1 plus numerous vacuolated macrophages. There was mild thickening of the leptomeninges with vacuolated leptomeningeal cells (Figure 4c). The leptomeninges contained many vacuolated macrophages, and there was likewise vacuolation of periarterial macrophages within the brain.
Foci of extramedullary haematopoiesis occurred in the liver and spleen involving both erythroid and myeloid precursors and particularly megakaryocytes in the latter. Similarly, increased haematopoiesis was observed in rib bones.
Enzymology and molecular genetic studies
Arylsulphatase B activity in affected dog fibroblasts was 8.3 pmol/min/mg protein (units) compared with 1,049 and 1,968 units in the two control dogs; in liver, the comparative figures were 0.1 vs. 58.4 and 80.5 units in controls. Sulphamidase activity in the affected dog fibroblasts was 28 pmol/min/mg protein being similar to 33 and 72 units noted in the two controls. This indicated that the deficient activity of arylsulphatase B noted was not due to multiple sulphatase deficiency and supported a diagnosis of MPS VI.
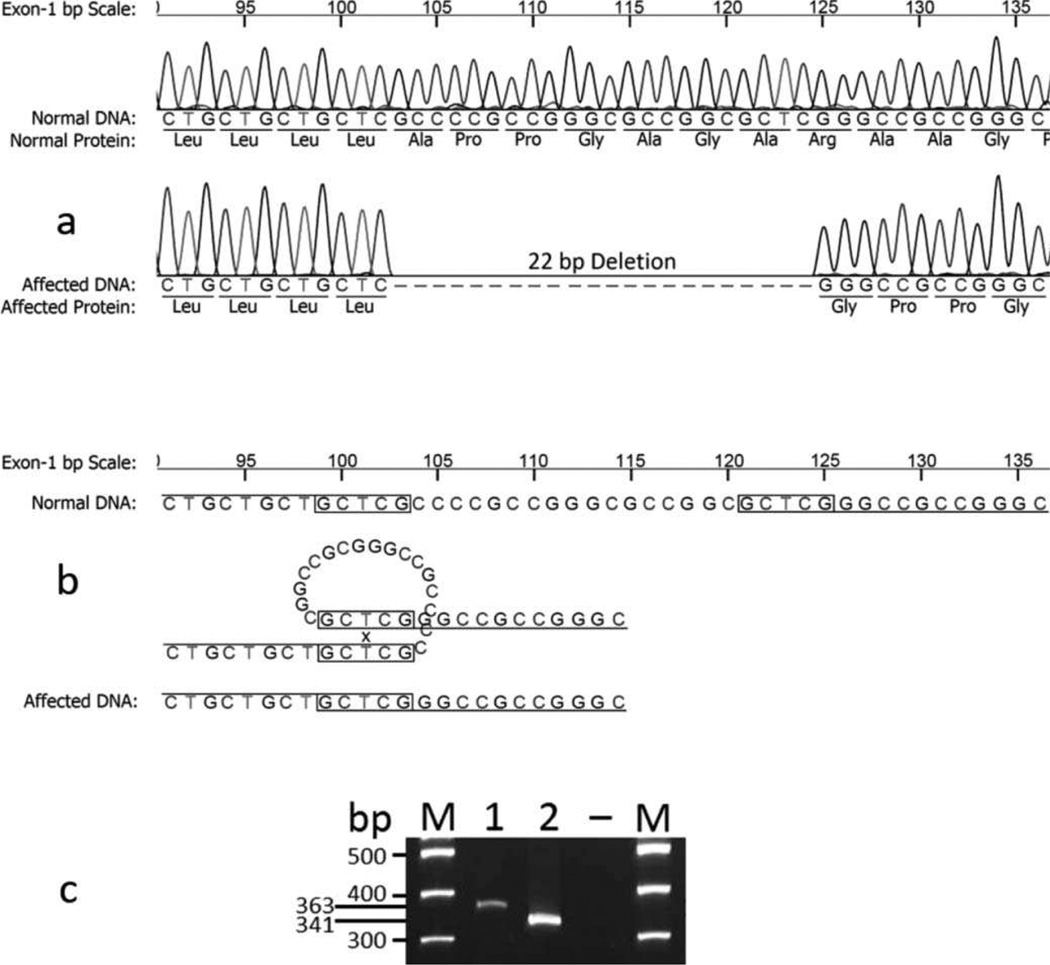
An initial screening of the affected dog’s DNA was reported by the laboratory as not carrying either of two known canine mutations in the MPS VI gene of Miniature Pinschers and Miniature Schnauzers, respectively. The DNA sequence for the arylsulphatase B gene was then investigated. When compared with the normal canine sequences, a 22 bp deletion was found in exon 1 (c.103_124del) from the affected dog (Figure 5a). The deletion was likely the result of crossing-over and looping at the 5 bp direct repeats (GCTCG) which are 17 bp apart in the normal sequence (Figure 5b). Due to two direct repeats, the precise location of this deletion could not be absolutely determined, but the effect on the protein remains the same. This mutation causes a frame-shift which results in a premature stop codon at position p.108 (translated from exon 2), and therefore, a truncated protein. Due to the large deletion, the mutant and normal amplicons were readily being separated by gel electrophoresis (Figure 5c).
Figure 5.
(a) The sequencing tracings and deduced nucleotide and amino acid sequences of part of exon 1 of arylsulphatase B gene from a control dog compared with those of a Miniature Poodle-like dog, diagnosed with mucopolysaccharidosis type VI; the latter shows a 22 base pair deletion. (b) The deletion is likely the result of a crossing-over and looping at the 5 base pair direct repeats (GCTCG) which are 17 base pair apart in the normal sequence. (c) Gel electrophoresis of the amplicons that differentiate the DNA fragment from the control dog (lane 1) and the 22 base pair smaller DNA fragment in the affected Poodle (lane 2). M = marker lanes; – is blank lane.
Based upon the Wisdom pure breed-mixed breed test, this dog’s DNA was reported to be that of a cross between a Miniature and Toy Poodle.
Discussion
On initial examination, a presumptive diagnosis of a MPS lysosomal storage disease was made on the basis of the metachromatic Alder-Reilly-type inclusions in leucocytes, corneal opacities and joint abnormalities. MPS I, MPS VI and MPS VII were considered the more likely diseases. Each of the above MPS disorders has been described in dogs; MPS I in a Plott hound (Shull et al. 1984), Plott hound × Beagle cross (Dickson et al. 2008), a Rottweiler and a Boston terrier (Haskins et al. 2006); MPS VI in Miniature Pinschers (Neer et al. 1995), Miniature Schnauzer, Cheasapeake Bay Retrievers and Welsh Corgis (Haskins and Giger 2008), and MPSV II in mixed breed dogs (Haskins et al. 1984) and German Shepherd dogs (Dombrowski et al. 2004). MPS VI has also been well characterised in Siamese and domestic shorthair cats (reviewed in Sewell et al. 2007; Haskins and Giger 2008).
Neither MPS, nor any other lysosomal storage disorder, has been previously described in Poodles. The dwarfism, generalised skeletal deformities from puppyhood and corneal opacities seen in the dog described here are most characteristic of MPS VI (Neer et al. 1995). The mild regenerative anaemia and reactive haemopoiesis were not thought to be related to this primary MPS disease and remained unexplained.
Much of the pathogenesis of MPS diseases is due to the accumulation of GAG in many tissues resulting in distorted growth or function. The lesions reported above were similar to, though more severe than those in Miniature Pinschers (Neer et al. 1995). The vertebral body shortening, epiphyseal dysplasia throughout the skeleton, hypoplasia of the odontoid process and joint laxities contributed most to the clinical signs in this dog. Corneal opacities are another characteristic result of GAG accumulation in the lysosomes of corneal stromal cells. These, and MPS associated thickening and distortion of heart valves, are also recorded in Miniature Pinschers (Neer et al. 1995).
There was no major primary neurological disease evident. Secondary Wallerian-type degeneration in hind-brain, proximal cervical cord and caudal bulbar nerves was attributed to mild trauma associated with the abnormalities arising from hypoplasia of the odontoid process. The latter is a common abnormality in those mucopolysaccharidoses with severe skeletal lesions and is frequently associated with degenerative neuropathy of the underlying nervous tissue (Barnes 2008).
Examination of blood smears is a simple screening test for some, but not all, storage diseases, as abnormal vacuoles, or accumulation of granules, may be found in different types of leucocytes and even platelets. However, these helpful diagnostic features may be overlooked, or misinterpreted as basophils, toxic neutrophils, or mast cells. If the inclusions in leucocytes are metachromatic, as was observed in this case, this is diagnostic for an MPS disorder. Although Leishman’s and Diff-Quik are both Romanowsky-type stains, their ability to stain the inclusions differed. The neutrophil inclusions were not obvious with the Diff-Quik stain in the case reported here, and the diagnosis could easily have been missed if this was the only stain used. The urinary Berry MPS spot test is another screening test to detect animals with MPS, but not other lysosomal storage disorders, with the possible exception of GM1 gangliosidosis. However, very young animals may normally have a slightly positive urinary Berry test result (Chih-Kuang et al. 2002).
A provisional morphological diagnosis of MPS VI was further supported by the pattern of dermatan sulphate in urine. It was confirmed by demonstrating negligible arylsulphatase B activity in liver and skin fibroblasts, with the exclusion of multiple sulphatase deficiency by demonstrating a similar level of sulphamidase activity in both affected and control cultured fibroblasts.
Direct sequencing of the arylsulphatase B gene identified a novel and major 22 bp deletion in exon 1, likely due to a unique crossing over. The resulting frame-shift not only changes the consequent amino acid sequence, but also leads to a premature stop codon in exon 2. Thus, this mutation predicts a severely truncated arylsulphatase B enzyme protein and consequently a deficiency of enzyme activity, as demonstrated in liver and fibroblasts from this dog. The deletion is flanked by completely conserved regions of the arylsulphatase B gene among primates and carnivores. It differs from two other known mutations for the MPS VI gene in Miniature Pinschers and Miniature Schnauzers (unpublished data) for which commercial tests are available. In human patients with MPS VI, at least 83 mutations were described with a clear correlation between genotype and urinary levels of GAG that can be used to predict clinical outcome. Some of these mutations are deletions in exon 1 with severe phenotypes similar to those in the dog described here (Karageorgos et al. 2007).
As relevant breeding information was not available, designation of this dog’s breed as Miniature Poodle-type was initially based on phenotype, including the characteristic Poodle coat, but because of its malformations and associated stunting, this was uncertain. The commercially available molecular Wisdom pure breed-mixed breed test reported this dog was a cross between a Miniature and Toy Poodle that supports the observed phenotype. The principles underlying the test have been published (Parker et al. 2004) and are set out in US patent 7729863 (Ostrander et al. 2010). It is able to analyse both alleles and so identify breed identities of cross-bred dogs.
Currently there is no information as to how wide spread this arylsulphatase B mutation is in Miniature or Toy Poodles in New Zealand, or elsewhere. However, the discovered mutation has allowed development of a simple molecular screening test that can discriminate both affected and heterozygous animals from normal ones, and which could be used to control this disorder in association with appropriate breeding strategies. Similar screening has revealed a wide distribution of MPS VI in Miniature Pinschers (U Giger, unpublished data) and Siamese cats (Crawley et al. 2003) allowing control of this disease by breeders. The MPS VI mutation in Miniature Schnauzers is very rare (U Giger, unpublished data).
This is the first report of MPS VI, or any other form of MPS in Miniature or Toy Poodles. It is likely that other cases have, or will be found in this, or related breeds. The congenital nature of the disorder is likely to lead to euthanasia of malformed pups without further investigation, so it could be more widespread than indicated by this single case. This report alerts veterinarians to a potential breed-related genetic problem and the availability of a genetic test to control it. This form of MPS VI is not suitable as an animal model of related human disorders.
Acknowledgements
The authors would like to acknowledge Professor Keith Thompson (IVABS) for Figure 1, Dr. Jianyu Chen (Manawatu Microscopy and Imaging Centre, Massey University, Palmerston North, New Zealand) for preparation of epoxy resin sections, Mrs E. Luxton (IVABS, Massey University) for preparation of the paraffin sections and Mrs Gloria Owens, (Metabolic Laboratory, Genetics and Molecular Pathology, SA Pathology, Adelaide) for the glycosaminoglycan analysis of urine. We thank them for their contributions to this investigation. The molecular genetic investigation was supported by a grant from the National Institutes of Health (NIH-RR02512).
Glossary
- bp
Base pair
- GAG
Glycosaminoglycan(s)
- MPS
Mucopolysaccharidosis
References
- Auclair D, Hopwood JJ, Brooks DA, Lemontt JF, Crawley AC. Replacement therapy in mucopolysaccharidosis type VI: advantages of early onset of therapy. Molecular Genetics and Metabolism. 2003;78:163–174. doi: 10.1016/s1096-7192(03)00007-6. [DOI] [PubMed] [Google Scholar]
- *.Barnes PD. Neuroimaging of the spine and spinal neuraxia in childhood. In: Kim DH, Betz RR, Huhn SL, Newton PO, editors. Surgery of the Pediatric Spine. New York, USA: Thieme Medical Publishers Inc; 2008. pp. 23–48. [Google Scholar]
- Chih-Kuang C, Shuan-Pei L, Shyue-Jye L, Tuen-Jen W. MPS screening methods, the Berry spot and acid turbidity tests, cause a high incidence of false-negative results in Sanfilippo and Morquio syndromes. Journal of Clinical Laboratory Analysis. 2002;16:253–258. doi: 10.1002/jcla.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley AC, Niedzielski KH, Isaac EL, Davey RC, Byers S, Hopwood JJ. Enzyme replacement therapy from birth in a feline model of mucopolysaccharidosis. Journal of Clinical Investigation. 1997;99:651–662. doi: 10.1172/JCI119208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley AC, Muntz FH, Haskins ME, Jones BR, Hopwood JJ. Prevalence of mucopolysaccharidosis type VI in Siamese cats. Journal of Veterinary Internal Medicine. 2003;17:495–498. doi: 10.1111/j.1939-1676.2003.tb02470.x. [DOI] [PubMed] [Google Scholar]
- Dickson P, Peinovich M, McEntee M, Lester T, Le S, Krieger A, Manuel H, Jabagat C, Passage M, Kakkis ED. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccaridosis. Journal of Clinical Investigation. 2008;118:2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrowski S, Carmichael KP, Wang P, O’Malley TM, Haskins ME, Giger U. Mucopolysaccharidosis type VII in a German Shepherd dog. Journal of American Veterinary Medical Association. 2004;224:553–557. doi: 10.2460/javma.2004.224.553. [DOI] [PubMed] [Google Scholar]
- *.Haskins M, Giger U. Kaneko JJ, Harvey JW, Bruss ML. Clinical Biochemistry of Domestic Animals. Burlington, USA: Elsevier Inc; 2008. Lysosomal storage diseases; pp. 731–749. [Google Scholar]
- Haskins ME, Desnick RJ, DiFerrante N, Jezyk PF, Patterson DF. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis type VII. Pediatric Research. 1984;18:980–984. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- *.Haskins ME, Giger U, Patterson DF. Animal models of lysosomal storage diseases: their development and clinical relevance. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 years of FOS. Oxford, England: Oxford PharmaGenesis; 2006. pp. 51–61. [PubMed] [Google Scholar]
- Hein LK, Meikle PJ, Dean CJ, Bockmann MR, Auclair D, Hopwood JJ, Brooks DA. Development of an assay for the detection of mucopolysaccharidosis VI patients using dried blood spots. Clinica Chimica Acta. 2005;353:67–74. doi: 10.1016/j.cccn.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Hopwood JJ, Elliott H. Diagnosis of Sanfilippo type A syndrome by estimation of sulfamidase activity using a radiolabelled tetrasaccharide substrate. Clinica Chimica Acta. 1982;123:241–250. doi: 10.1016/0009-8981(82)90168-1. [DOI] [PubMed] [Google Scholar]
- Hopwood JJ, Harrison JR. High-resolution electrophoresis of urinary glycosaminoglycans: improved screening test for the mucopolysaccharidoses. Analytical Biochemistry. 1982;119:120–127. doi: 10.1016/0003-2697(82)90674-1. [DOI] [PubMed] [Google Scholar]
- Jolly RD, Walkley SU. Lysosomal storage diseases of animals: an essay in comparative pathology. Veterinary Pathology. 1997;34:527–548. doi: 10.1177/030098589703400601. [DOI] [PubMed] [Google Scholar]
- Karageorgos L, Brooks DA, Pollard A, Melville EL, Hein LK, Clements PR, Ketteridge D, Swiedler SJ, Beck M, Giugliani R, Harmatz P, Wraith JE, Guffon N, Teles EL, Miranda MCS, Hopwood JJ. Mutational analysis of 105 mucopolysaccharidosis type VI patients. Human Mutation. 2007;28:897–903. doi: 10.1002/humu.20534. [DOI] [PubMed] [Google Scholar]
- Neer TM, Dial SM, Pechman R, Wang P, Oliver JL, Giger U. Clinical vignette: mucopolysaccharidosis type VI in a miniature Pinscher. Journal of Veterinary Internal Medicine. 1995;9:429–433. doi: 10.1111/j.1939-1676.1995.tb03306.x. [DOI] [PubMed] [Google Scholar]
- *.Neufeld EF, Meuezner J. The mucopolysaccharidoses. In: Scriver R, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Bases of Inherited Disease. New York, USA: McGraw-Hill; 2001. pp. 3421–3452. [Google Scholar]
- *.Ostrander E, Kruglyak L, Parker HG, Kim LV, Stephens M, Malek TB, Sutter NB, Carlson S. Methods and materials for canine breed identification. 7729863. U.S. Patent. 2010
- Parker HC, Kim LV, Sutter NB, Carlson S, Lorentzen TD, Malek TB, Johnson GS, DeFrance HB, Ostrander EA, Kruglyak L. Genetic structure of the purebred domestic dog. Science. 2004;304:1160–1164. doi: 10.1126/science.1097406. [DOI] [PubMed] [Google Scholar]
- Rittie L, Fisher GJ. Isolation and culture of skin fibroblasts. Methods in Molecular Medicine. 2005;117:83–98. doi: 10.1385/1-59259-940-0:083. [DOI] [PubMed] [Google Scholar]
- Sewell AC, Haskins ME, Giger U. Inherited metabolic disease in companion animals: searching for nature’s mistakes. The Veterinary Journal. 2007;174:252–259. doi: 10.1016/j.tvjl.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull RM, Helman RG, Spellacy E, Constantopouplos G, Munger RJ, Neufeld EF. Morphologic and biochemical studies of canine mucopolysaccharidosis I. American Journal of Pathology. 1984;114:487–495. *Non-peer-reviewed