Figure 4.
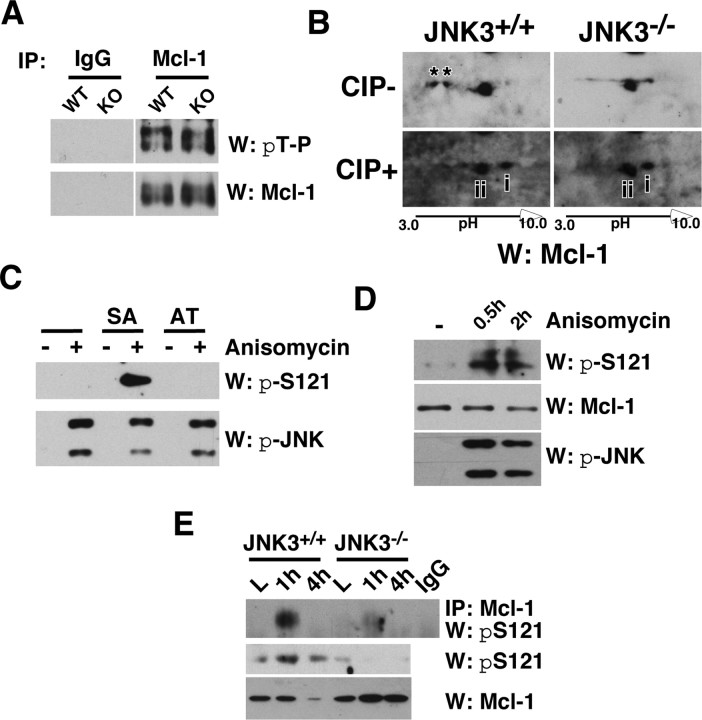
JNK3 phosphorylates Mcl-1 at S121 in vivo after injury. A, Mcl-1 was phosphorylated at T163P in the uninjured spinal cord regardless of JNK3 genotype. The uninjured lysates were subjected to immunoprecipitation with the control IgG or Mcl-1 antibody and probed in a Western blot with pThr-Pro antibody. The same blot was stripped and reprobed for Mcl-1 as a control. B, JNK3 phosphorylated Mcl-1 at an additional site after injury in vivo. Injury lysates collected at 1 dpi were subjected to two-dimensional electrophoresis and subsequent Western blot with Mcl-1, with and without CIP treatment. Note that Mcl-1 has two additional phosphorylated bands in JNK3+/+ but not in JNK3−/− mice without CIP treatment (asterisks), and the distance between unphosphorylated and partially phosphorylated Mcl-1 is greater in JNK3+/+ than in JNK3−/− mice after CIP treatment (compare the distance between i and ii). C, D, Specificity of the pS121P-Mcl-1 antibody. 293T cells were transfected with Mcl-1SA and Mcl-1AT mutants (C) or not (D). To phosphorylate the endogenous Mcl-1 or the mutants, we treated 293T cells with 50 ng/ml anisomycin for 30 min or 2 h. To inhibit degradation of phosphorylated Mcl-1, we also added 10 μm MG132 to cells before anisomycin treatment. Note that pS121P-Mcl-1 antibody detected Mcl-1SA, but not the Mcl-1AT mutant, and only when JNK was activated (C) and, similarly, the endogenous Mcl-1 only after anisomycin treatment (D). E, JNK3 phosphorylated Mcl-1 at S121P after injury. The injury lysates were subjected to immunoprecipitation with the control IgG or Mcl-1 antibody and probed in Westerns with the pS121P-Mcl-1 antibody. As controls, direct Western blots with pS121P-Mcl-1 and Mcl-1 antibodies also are shown. All of the experiments were repeated two to three times with similar results.