Abstract
Objective
To examine, in the light of the association between urban environment and poor mental health, whether urbanisation and neighbourhood deprivation are associated with analgesic escalation in chronic pharmacological pain treatment and whether escalation is associated with prescriptions of psychotropic medication.
Design
Longitudinal analysis of a population-based routine dispensing database in the Netherlands.
Setting
Representative sample of pharmacies, covering 73% of the Dutch nationwide medication consumption in the primary care and hospital outpatient settings.
Participants
449 410 patients aged 15–85 years were included, of whom 166 374 were in the Starter group and 283 036 in the Continuation group of chronic analgesic treatment.
Main outcome measure
Escalation of analgesics (ie, change to a higher level of analgesic potency, classified across five levels) in association with urbanisation (five levels) and dichotomous neighbourhood deprivation was analysed over a 6-month observation period.
Methods
Ordered logistic multivariate model evaluating analgesic treatment.
Results
In both Starter and Continuation groups, escalation was positively associated with urbanisation in a dose–response fashion (Starter group: OR (urbanisation level 1 compared with level 5): 1.24, 95% CI 1.18 to 1.30; Continuation group: OR 1.18, 95% CI 1.14 to 1.23). An additional association was apparent with neighbourhood deprivation (Starter group: OR 1.07, 95% CI 1.02 to 1.11; Continuation group: OR 1.04, 95% CI 1.01 to 1.08). Use of somatic and particularly psychotropic co-medication was associated with escalation in both groups.
Conclusions
Escalation of chronic analgesic treatment is associated with urban and deprived environments and occurs in a context of adding psychotropic medication prescriptions. These findings suggest that pain outcomes and mental health outcomes share factors that increase risk and remedy suffering.
Article summary
Article focus
We examined the hypothesis that chronic pharmacological pain treatment of hospital outpatients and patients in primary care would show escalation of analgesics in association with level of urbanisation and neighbourhood index of deprivation.
It was predicted that the highest levels of urbanisation and neighbourhood deprivation would be associated with escalation of analgesic treatment to more potent pain medication (eg, tramadol, morphine, methadone, etc).
Furthermore, we examined the hypothesis that prescriptions of psychotropic medication (eg, antidepressants, antipsychotics, mood stabilisers, etc) would be associated with escalation of analgesics in patients prescribed chronic analgesic treatment.
Key messages
Escalation of chronic analgesic treatment in persistent pain states is associated with urban environments and deprived neighbourhoods and occurs in a context of increased levels of psychotropic medication prescribing, suggesting that persistent pain outcomes are associated with area influences affecting mental health.
Broadening the pain agenda to an understanding which includes mental health perspectives will enhance understanding of central pain sensitisation and could minimise negative classical pain treatment outcomes, for instance failed back surgery or negative opioid-associated consequences, especially in patients with undetected mental disorders.
From a public health and clinical perspective, a more effective treatment of persistent pain, including treatment of psychiatric comorbidity, may save costs. A new focus in populations with persistent pain states on early recognition and treatment of mental health problems may be cost-effective and also represents an area of unmet clinical need.
Strengths and limitations of this study
We have been able to use a large general practice–related dispensing data set to examine contextual influences on pain medication escalation. To our knowledge, this is the first study that revealed clear associations of persistent pain outcomes with urban environments in a context of psychotropic medication prescriptions. The results clearly echo findings of unconfounded higher rates of poor mental health in areas of higher levels of urbanisation and greater neighbourhood deprivation and emphasise the need to recognise mental health conditions in persistent pain states.
The results of the current study should be seen in the light of several limitations. The use of routine data rather than a targeted data collection could have caused more random error resulting in type II error. Unidentified confounding may have played a role, as randomisation was not possible and pre-design and post-design are sensitive to effects of unmeasured changes affecting outcome measures over time. Another limitation is the lack of outcomes other than urbanisation, psychotropic medication or somatic co-medication. For instance, there were no estimates regarding care consumption or illness-related sick leave. Changes in patient-related outcomes like illness severity, global functioning, quality of life and treatment satisfaction should also form part of prospective evaluations. The type of data used is subject to the possibility of ecological fallacy: people whose pharmacy is in a deprived or urban neighbourhood do not necessarily experience that level of deprivation or urbanicity. Furthermore, this study only collected data over a 12-month period. Affect and pain monitoring deserves longer evaluation. Finally, due to the study design, associations do not allow for causal inference.
Introduction
The validity of the well-known epidemiological association between urban environment and mental health1–3 is supported by work showing that urban living is associated with increased amygdala activity,4 a key region in the regulation of stress, affective experience and pain.5 6 Pain is the natural comorbid mental experience of somatic conditions.7 8 In turn, pain is strongly influenced by comorbid common mental disorders particularly affective disorders.9 10 Given evidence of urban impact on risk for common mental disorders,11 including psychiatric medication prescriptions,12 we hypothesised that pain outcomes, indexed through prescriptions, would be poorer in urban environments and disadvantaged urban neighbourhoods. Pain outcomes were examined at the level of primary care and specialist outpatient care and defined in two ways: (1) escalation of analgesic treatment (ie, prescription of more potent analgesics) and (2) co-prescription of psychotropic medication in addition to analgesic treatment.
Objective
We examined the hypothesis that chronic pharmacological pain treatment of hospital outpatients and patients in primary care would show escalation of analgesics in association with the level of urbanisation and neighbourhood index of deprivation. It was predicted that the highest levels of urbanisation and neighbourhood deprivation would be associated with escalation of analgesic treatment to more potent pain medication (eg, tramadol, morphine, methadone, etc). Furthermore, we examined the hypothesis that prescriptions of psychotropic medication (eg, antidepressants, antipsychotics, mood stabilisers, etc) would be associated with escalation or de-escalation of analgesics in patients prescribed chronic analgesic treatment. Study hypotheses were specified before inspection of the data.
Methods
Data collection
The investigation was carried out by analysing records pertaining to Dutch routine general practice and hospital outpatient treatment settings. Data were obtained from the IMS Health's longitudinal prescription database (Lifelink, affiliate Capelle ad IJssel, the Netherlands).13 This data source consists of anonymous longitudinal prescription records from a representative sample of pharmacies and dispensing general practitioners, covering 73% of the Dutch nationwide medication consumption of outpatients and primary care patients. The computerised medication-dispensing histories contain data regarding dispensed medications, type of prescriber, dispensing date, dispensed amount of medication, prescribed dosage and length of prescription. Data for each patient were anonymously and independently sampled without linkage of prescriptions to the same patient across pharmacies because patients in the Netherlands are usually loyal to a single pharmacy.14 Furthermore, research from the Dutch Foundation for Pharmaceutical Statistics (SFK) revealed that in the Netherlands, almost all patients make use of a pharmacy located in their area of living. Eighty-two per cent of patients are living in a radius of 3 km from their pharmacy.15 Potential bias caused by patients getting hospitalised, moving to another address or dying was minimised by studying chronic pharmacological pain treatment because there were dispensing records for these patients during the whole study period.
Patient groups
Patient selection started with the identification of chronic users of analgesic medication during a 6-month prescription period (hereafter: observation period). Chronic use was defined as in receipt of analgesic pharmacotherapy during at least two distinct moments covering an interval of at least 2 months. In order to track medication for other therapeutic indications (ie, psychotropic medication and pharmacotreatment for somatic disorders), patients were observed for a period of 6 months prior to initiation of analgesic treatment. Next, the cohort with chronic use of analgesics was divided into two groups. Starters were defined as patients who had not received any analgesics during the 6-month period prior to the observation period (hereafter: Starter group). Patients who continued with pain medication that was already prescribed in the 6 months before the observation period formed the second group (hereafter: Continuation group). The latter group consisted of all patients who had already received analgesics in the first month of the 6-month period prior to the observation period in order to define chronic analgesic treatment before observation. All data captured a calendar period from May 2008 to September 2009 (figure 1).
Figure 1.
Starter group and Continuation group of chronic analgesic treatment. Schedule of prescriptions (Rx) in the Starter group (top) and the Continuation group (bottom) of chronic analgesic treatment covering a 12-month period. Months 7–12 are the observation period; months 1–6 are the pre-observation period. Patients in the Continuation group received the first prescription of analgesics in month 1 of the pre-observation period; there was no follow-up whether analgesics were continued over the entire 6-month interval prior to the observation period. The Starter group did not use any analgesics during the 6-month interval prior to the observation period.
Data were obtained from the LRx database from months 1–12 as depicted in figure 1. Use of other medications (eg, psychotropic medication and medication for a broad spectrum of somatic conditions) was collected for all patients as well, covering the period of 12 months, consisting of (1) the pre-observation period (months 1–6) and (2) the observation period (months 7–12).
Escalation of pharmacological pain treatment
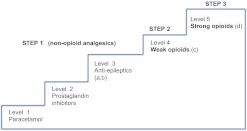
All individual prescriptions of analgesics were observed for each patient in both the Starter and the Continuation groups during the observation period and during the 6 months prior to the observation period. At each dispensing date, analgesics were classified a priori in five levels in order of analgesic potency (figure 2). Five escalation levels were provided, based on a minor adaptation of the three-step WHO analgesic ladder.16 Levels 5 and 4 are identical to WHO steps 3 (strong opioids) and 2 (weak opioids), respectively. WHO step 1 (non-opioid analgesics) was refined in order to enable further and clinically relevant differentiation between non-opioid analgesics (level 1: paracetamol, level 2: prostaglandin inhibitors and level 3: anticonvulsants).16–20 Furthermore, anti-epileptics were divided in anticonvulsants predominantly prescribed in pain conditions (level 3a: gabapentin and pregabalin) and anticonvulsants with best evidence for epilepsy treatment (level 3b: carbamazepine, valproic acid and lamotrigine).19–21 In order to avoid prescription for indications of mood stabilisation or epilepsy, the latter group was classified at level 3b only if prescribed in combination with analgesic medication at level 1 or 2 (ie, pain indication) (figure 2).
Figure 2.
Five levels of analgesic potency, modified from the WHO analgesic ladder.16 Level 1 (ie, lowest potency) to level 5 (ie, highest potency). (a) Gabapentin and pregabalin in the absence of other anti-epileptic drugs. (b) Carbamazepine, valproic acid and lamotrigine in combination with medication at level 1 or 2. (c) Tramadol and codeine. (d) Methadone, oxycodone, hydromorphone, morphine, buprenorphine, fentanyl, sufentanil and pethidine.
Confirmation of escalation was based on the comparison of analgesic potency at the first dispensing day and the last dispensing day within the observation period.
The comparison of the first and the last prescription of analgesics resulted in the following categories of analgesic escalation: neutral (ie, no change of analgesic potency), escalation in analgesic treatment (ie, change to a higher level of analgesic potency) or de-escalation in pharmacological pain treatment (ie, change to a lower level of analgesic potency) (table 1).
Table 1.
Sample, stratified by urbanisation and neighbourhood deprivation
| Type of patient | Urbanisation level | Deprived neighbourhood |
% Within deprived neighbourhood | |||
| No (patients) | No (%) | Yes (patients) | Yes (%) | |||
| Starter group | 1 | 34 662 | 76.3 | 10 796 | 23.7 | 86.5 |
| 2 | 48 673 | 96.6 | 1689 | 3.4% | 13.5 | |
| 3 | 31 107 | 100.0 | 0 | 0.0 | – | |
| 4 | 28 283 | 100.0 | 0 | 0.0 | – | |
| 5 | 11 164 | 100.0 | 0 | 0.0 | – | |
| Total | 153 889 | 92.5 | 12 485 | 7.5 | 100.0 | |
| Continuation group | 1 | 59 714 | 76.2 | 18 644 | 23.8 | 85.5 |
| 2 | 81 406 | 96.3 | 3155 | 3.7 | 14.5 | |
| 3 | 50 853 | 100.0 | 0 | 0 | – | |
| 4 | 48 511 | 100.0 | 0 | 0 | – | |
| 5 | 20 754 | 100.0 | 0 | 0 | – | |
| Total | 261 237 | 92.3 | 21 799 | 7.7 | 100.0 | |
| Total patients | 415 126 | 92.4 | 34 284 | 7.6 | ||
The sample is described in absolute numbers for the Starter and the Continuation groups, stratified by living in an urbanised area (levels 1–5) and a dichotomous measure of neighbourhood deprivation. Furthermore, in the last column, tabulation is presented for living in a deprived neighbourhood as a function of level of urbanisation (eg, in the Starter group, 86.5% of the sample living in deprived neighbourhoods lived in an area with urbanisation level 1).
If patients received several analgesics on the same day, both the highest and the second highest level of analgesic potency were included in the analyses in order to define escalation categories (eg, a change from level 5 plus level 2 to level 5 plus level 3 indicating that escalation had occurred).
Determinants of escalation in analgesic treatment
Three groups of variables hypothesised to act as mediators or confounders were included in the analyses. The first group were patient characteristics such as sex (0=male, 1=female), age (in years) and the location of patient's pharmacy (defined by postal code). The latter variable defined the level of urbanisation following the definition of the Dutch Central Bureau of Statistics (CBS). Conform previous work, and in line with the classification developed by CBS, level of urbanisation was defined as the number of addresses relative to area surface.22 Level 1 (ie, highest level of urbanisation) consists of more than 2500 addresses/km2 (level 2=1500–2500 addresses/km2, level 3=1000–1500 addresses/km2, level 4=500–1000 addresses/km2). Level 5 (ie, rural environment) consists of <500 addresses/km2, described in more detail elsewhere.23 Moreover, neighbourhood deprivation was defined dichotomously (0= no, 1= yes). The dichotomous measure of neighbourhood deprivation was developed by the Netherlands Institute of Research in Healthcare (NIVEL) using socioeconomic indicators such as unemployment rate, average income, population density and ethnic variation. On the basis of empirical research in the Netherlands, NIVEL's neighbourhood deprivation index (NDI) is calculated as follows: NDI = ((ln percentage unemployed people − 3.0236)/0.37 706) − ((ln average income − 2.8641)/0.14 441) + ((ln population density − 7.0132)/1.06 699) + ((ln percentage people of ‘non-western’ ethnicity)/1.11 147). NDIs were expressed continuously by NIVEL from low to high. Furthermore, NIVEL defined a dichotomous measure of deprivation at a cut-off of 5.5% (ie, 885 000 people) in order to assess trends in the proportion of the Dutch population inhabiting an area with the highest NDI and for use in epidemiological research.24 Healthcare professionals receive higher levels of funding for their services in these deprived areas.25 Neighbourhood deprivation was associated with level of urbanisation: 86% of the sample living in deprived neighbourhoods lived in an area with the highest level of urbanisation. The other patients (14%) living in deprived neighbourhoods lived in an area with the second highest level of urbanisation. The majority (76%) of those living in an area of the highest level of urbanisation did not live in a deprived neighbourhood (table 1).
Furthermore, psychotropic co-medication was classified into its different classes, and somatic co-medication was similarly grouped in 10 classes (ACE inhibitors and angiotensin II inhibitors; antidiabetics; β-blockers; calcium antagonists; functional bowel drugs; laxatives; migraine medication; respiratory medication; steroid antiphlogistics; stomach protectors) (tables 1–3). In the Starting group, occurrence of co-medication was time coded at three levels according to the day of first occurrence (ie, co-medication prescription before start with analgesics at the same day or after start of analgesic treatment) (table 3). In the Continuation group, occurrence of co-medication was recorded dichotomously (presence/absence) since it was impossible to distinguish occurrence of co-medication as before or at start of analgesic treatment (table 4).
Table 2.
Baseline characteristics of the patient population with chronic analgesic treatment
| Starter group |
Continuation group |
|||||||
| Deprived | Urbanicity* | Urbanicity | Non-deprived | Deprived | Urbanicity | Urbanicity | Non-deprived | |
| Neighbourhoods | 1 | 2–5 | Neighbourhoods | Neighbourhoods | 1 | 2–5 | Neighbourhoods | |
| Patients (absolute) | 12 485 | 45 458 | 120 916 | 153 889 | 21 799 | 78 358 | 204 678 | 261 237 |
| Change in analgesics† | ||||||||
| De-escalation | 13.3 | 12.1 | 10.4 | 10.7 | 13.2 | 12.5 | 11.2 | 11.4 |
| Neutral | 70.1 | 71.6 | 74.5 | 74.0 | 70.0 | 71.4 | 73.7 | 73.3 |
| Escalation | 16.5 | 16.3 | 15.1 | 15.3 | 16.8 | 16.1 | 15.1 | 15.3 |
| Gender | ||||||||
| Male | 39.8 | 39.3 | 40.3 | 40.0 | 36.7 | 35.2 | 34.6 | 34.6 |
| Female | 60.2 | 60.7 | 59.7 | 60.0 | 63.3 | 64.8 | 65.4 | 65.4 |
| Age (years) | ||||||||
| 15–25 | 6.3 | 6.1 | 6.5 | 6.4 | 1.8 | 1.6 | 1.8 | 1.7 |
| 26–40 | 23.8 | 19.2 | 16.3 | 16.6 | 14.0 | 10.8 | 9.7 | 9.7 |
| 41–65 | 50.0 | 49.6 | 50.9 | 50.6 | 56.9 | 53.4 | 51.8 | 51.9 |
| 65–85 | 19.8 | 25.2 | 26.2 | 26.4 | 27.3 | 34.2 | 36.7 | 36.7 |
| First analgesics | ||||||||
| Level 1 | 3.2 | 3.9 | 4.0 | 4.0 | 3.0 | 3.8 | 4.1 | 4.1 |
| Level 2 | 64.8 | 66.6 | 72.6 | 71.4 | 47.8 | 47.1 | 53.6 | 52.1 |
| Level 3 | 2.2 | 2.5 | 2.6 | 2.6 | 6.4 | 8.1 | 8.9 | 8.8 |
| Level 4 | 27.4 | 24.2 | 18.3 | 19.3 | 36.1 | 33.9 | 27.6 | 28.8 |
| Level 5 | 2.4 | 2.9 | 2.6 | 2.7 | 6.8 | 7.2 | 5.9 | 6.2 |
| Level 4/5 | 29.8 | 27.0 | 20.8 | 21.9 | 42.8 | 41.1 | 33.5 | 35.0 |
| Last analgesics | ||||||||
| Level 1 | 3.4 | 3.9 | 4.0 | 4.0 | 3.4 | 4.1 | 4.3 | 4.3 |
| Level 2 | 61.9 | 63.0 | 68.4 | 67.4 | 44.5 | 44.1 | 50.1 | 48.8 |
| Level 3 | 3.0 | 3.3 | 3.4 | 3.4 | 7.0 | 8.4 | 9.2 | 9.1 |
| Level 4 | 27.6 | 24.6 | 18.9 | 19.9 | 36.8 | 34.5 | 28.4 | 29.6 |
| Level 5 | 4.1 | 5.2 | 5.3 | 5.3 | 8.3 | 9.0 | 7.9 | 8.2 |
| Level 4/5 | 31.7 | 29.8 | 24.2 | 25.2 | 45.1 | 43.4 | 36.3 | 37.7 |
| Concomitant medication‡ | ||||||||
| Any concomitant medication | 78.8 | 79.0 | 77.3 | 77.7 | 89.3 | 89.6 | 88.0 | 88.4 |
| Migraine medication | 3.9 | 3.6 | 3.8 | 3.7 | 5.9 | 5.2 | 5.1 | 5.1 |
| Any psychotropic medication | 35.1 | 36.6 | 34.8 | 35.3 | 51.6 | 53.2 | 50.2 | 51.0 |
| Sedatives | 27.0 | 29.0 | 27.4 | 27.9 | 41.5 | 43.4 | 40.6 | 41.4 |
| Mood stabilisers | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 |
| Antipsychotics total | 4.1 | 3.9 | 3.1 | 3.2 | 6.7 | 6.1 | 4.9 | 5.1 |
| Antipsychotics 2nd generation | 2.4 | 2.2 | 1.5 | 1.6 | 4.1 | 3.5 | 2.5 | 2.7 |
| Antipsychotics classic | 2.0 | 2.1 | 1.8 | 1.8 | 3.2 | 3.2 | 2.7 | 2.8 |
| Bupropion | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 |
| MAO inhibitors | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 |
| TCA | 5.2 | 5.0 | 5.0 | 5.0 | 9.5 | 9.4 | 9.6 | 9.6 |
| Other antidepressants§ | 2.4 | 2.0 | 1.6 | 1.7 | 4.0 | 3.3 | 2.7 | 2.8 |
| SNRI | 2.6 | 2.4 | 2.2 | 2.2 | 4.5 | 4.0 | 3.5 | 3.6 |
| SSRI | 7.5 | 7.0 | 6.4 | 6.5 | 10.3 | 10.0 | 9.0 | 9.2 |
| Psychostimulants | 0.4 | 0.5 | 0.5 | 0.5 | 0.3 | 0.5 | 0.4 | 0.5 |
| Any somatic medication | 72.3 | 72.6 | 71.0 | 71.4 | 83.0 | 82.9 | 81.7 | 81.9 |
| Cardiovascular medication¶ | 30.9 | 31.4 | 30.8 | 30.9 | 35.2 | 34.9 | 34.9 | 34.9 |
| Other Somatic medication** | 65.2 | 65.5 | 64.0 | 64.3 | 76.9 | 76.7 | 75.4 | 75.6 |
Patient characteristics are presented as percentages (eg, age, gender, level of analgesic treatment, change in analgesic treatment (eg, escalation, de-escalation and neutral development of prescriptions) and concomitant medication). Absolute patient numbers are presented for the Starter and the Continuation groups of chronic analgesic treatment concerning level of urbanicity and for neighbourhood deprivation.
Urbanicity = urbanisation (level 1= highest level of urbanisation; level 5= rural environment).
Change in pain medication from the first to the last prescription (neutral = no change in level of potency, escalation = change to a higher level of analgesic potency, de-escalation = change to a lower level of analgesic potency).
Concomitant drug use, observed during a period of 12 months.
Other than bupropion, MAO inhibitors, SNRI, SSRI and TCA.
Cardiovascular medication: β-blocker, calcium antagonist, ACE inhibitor, angiotensin II inhibitor.
Gastrointestinal medication: antidiabetics, steroid antiphlogistics, respiratory medication.
TCA, tricyclic antidepressants; SNRI, selective noradrenaline–serotonin reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors.
Table 3.
Associations with escalation in pharmacological pain treatment for the Starter group of chronic analgesic treatment
| Exposure | Adjusted OR | 95% CI |
|
| Lower | Upper | ||
| Analgesics* | |||
| Level 1 | 58.23 | 53.60 | 63.27 |
| Level 2 | 17.92 | 16.75 | 19.16 |
| Level 3 | 4.66 | 4.23 | 5.14 |
| Level 4 | 1.36 | 1.27 | 1.45 |
| Level 5 | Reference | – | – |
| Gender | |||
| Female | 0.97 | 0.95 | 0.99 |
| Male | Reference | 0.00 | 0.00 |
| Age (years) | |||
| 15–25 | 0.73 | 0.69 | 0.77 |
| 26–40 | 0.81 | 0.78 | 0.84 |
| 41–65 | 0.87 | 0.85 | 0.90 |
| 66–85 | Reference | – | – |
| Urbanisation† | |||
| 1 | 1.24 | 1.18 | 1.30 |
| 2 | 1.16 | 1.11 | 1.22 |
| 3 | 1.11 | 1.06 | 1.17 |
| 4 | 1.07 | 1.02 | 1.13 |
| 5 | Reference | – | – |
| Deprived neighbourhood | |||
| Yes | 1.07 | 1.02 | 1.11 |
| No | Reference | – | – |
| SNRI | |||
| Before start of analgesics‡ | 1.05 | 0.96 | 1.14 |
| Same start date | 1.28 | 0.97 | 1.69 |
| After analgesics started | 1.26 | 1.09 | 1.45 |
| None | Reference | – | – |
| SSRI | |||
| Before start of analgesics | 0.97 | 0.92 | 1.02 |
| Same start date | 0.97 | 0.83 | 1.15 |
| After analgesics started | 1.07 | 0.97 | 1.18 |
| None | Reference | – | – |
| TCA | |||
| Before start of analgesics | 1.23 | 1.15 | 1.32 |
| Same start date | 1.32 | 1.12 | 1.54 |
| After analgesics started | 2.19 | 2.03 | 2.36 |
| None | Reference | – | – |
| Other antidepressants | |||
| Before start of analgesics | 1.03 | 0.93 | 1.15 |
| Same start date | 0.93 | 0.71 | 1.21 |
| After analgesics started | 1.22 | 1.06 | 1.42 |
| None | Reference | – | – |
| Antipsychotics | |||
| Before start of analgesics | 0.92 | 0.85 | 1.01 |
| Same start date | 0.69 | 0.58 | 0.83 |
| After analgesics started | 2.42 | 2.18 | 2.67 |
| None | Reference | – | – |
| Mood stabilisers | |||
| Before start of analgesics | 1.40 | 1.10 | 1.79 |
| Same start date | 0.91 | 0.43 | 1.89 |
| After analgesics started | 0.71 | 0.39 | 1.31 |
| None | Reference | – | – |
| Sedatives | |||
| Before start of analgesics | 1.24 | 1.20 | 1.28 |
| Same start date | 1.25 | 1.18 | 1.33 |
| After analgesics started | 1.82 | 1.74 | 1.89 |
| None | Reference | – | – |
| Cardiovascular drugs | |||
| Before start of analgesics | 1.16 | 1.13 | 1.19 |
| Same start date | 0.86 | 0.79 | 0.95 |
| After analgesics started | 1.35 | 1.26 | 1.45 |
| None | Reference | – | – |
| Other somatic drugs | |||
| Before start of analgesics | 1.25 | 1.22 | 1.29 |
| Same start date | 1.11 | 1.07 | 1.15 |
| After analgesics started | 1.19 | 1.15 | 1.23 |
| None | Reference | – | – |
| Migraine medication | |||
| Before start of analgesics | 0.83 | 0.77 | 0.89 |
| Same start date | 0.95 | 0.78 | 1.17 |
| After analgesics started | 0.91 | 0.81 | 1.02 |
| None | Reference | – | – |
Escalation = change to a higher level of analgesic potency, level 5= highest level.
1= highest level of urbanisation, 5= rural environment.
Starting date of medication (before, at the same day or after start of analgesics).
TCA, tricyclic antidepressants; SNRI, selective noradrenaline–serotonin reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors.
Table 4.
Associations with escalation in pharmacological pain treatment for the Continuation group of chronic analgesic treatment
| Exposure | Adjusted OR | 95% CI |
|
| Lower | Upper | ||
| Analgesics* | |||
| Level 1 | 16.00 | 15.20 | 16.85 |
| Level 2 | 7.87 | 7.59 | 8.16 |
| Level 3 | 3.14 | 3.00 | 3.28 |
| Level 4 | 1.55 | 1.50 | 1.61 |
| Level 5 | Reference | – | – |
| Gender | |||
| Female | 0.96 | 0.94 | 0.98 |
| Male | Reference | – | – |
| Age (years) | |||
| 15–25 | 0.91 | 0.85 | 0.97 |
| 26–40 | 0.98 | 0.95 | 1.01 |
| 41–65 | 0.99 | 0.97 | 1.01 |
| 66–85 | Reference | – | – |
| Urbanisation† | |||
| 1 | 1.18 | 1.14 | 1.23 |
| 2 | 1.14 | 1.10 | 1.17 |
| 3 | 1.08 | 1.04 | 1.12 |
| 4 | 1.05 | 1.01 | 1.09 |
| 5 | Reference | – | – |
| Deprived neighbourhood | |||
| Yes | 1.04 | 1.01 | 1.08 |
| No | Reference | – | – |
| SNRI | 1.19 | 1.02 | 1.40 |
| SSRI | 1.03 | 1.004 | 1.07 |
| TCA‡ | 1.19 | 1.06 | 1.32 |
| Other antidepressants | 1.08 | 1.03 | 1.14 |
| Antipsychotics | |||
| Total | 1.24 | 1.08 | 1.43 |
| 1st generation | 1.01 | 0.88 | 1.15 |
| 2nd generation | 0.80 | 0.70 | 0.91 |
| Mood stabilisers | 0.97 | 0.85 | 1.10 |
| Sedatives | 1.31 | 1.29 | 1.34 |
| Migraine | 0.95 | 0.91 | 0.99 |
| Cardiovascular drugs | 1.12 | 1.10 | 1.14 |
| Other somatic drug classes | 1.12 | 1.10 | 1.14 |
Escalation = change to a higher level of analgesic potency, level 5= highest level.
1= highest level of urbanisation, 5= rural environment.
TCA total (fully saturated model: OR 1.19, 95% CI 1.06 to 1.32; backward elimination model: OR 1.33, 95% CI 1.29 to 1.36).
TCA, tricyclic antidepressants; SNRI, selective noradrenaline–serotonin reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors.
Statistical analysis
First, we analysed the pattern of (de-) escalation in analgesic treatment by means of an ordered logistic multivariable regression model with adjusted ORs (and 95% CI) using SAS V.9.26 Statistical significance for the model was defined at conventional α of 0.05. The dependent variable in this model was the development of a patient's analgesic treatment (de-escalation, neutral, escalation). Independent variables, entered simultaneously in the model, were demographic characteristics, neighbourhood deprivation, and urbanisation, use of psychotropic medication and use of somatic medication. In the Starter group, we also included first occurrence of co-medication. The modelling strategy was to build, first, a fully saturated model (including all variables) in order to avoid missing relevant information by leaving out non-significant variables. Second, backward elimination was carried out to find the best model fit.
Models for the Starter and Continuation groups were run separately, given different sample selection criteria. The ordered logistic multivariable regression model was chosen above the multinomial model, as the latter does not consider the natural order in our data regarding development of chronic pain treatment, ranging from de-escalation to neutral to escalation. Proportional odds were assumed in the models of escalation and de-escalation of analgesic treatment and analyses inspected for violation of this assumption. Test on the proportional odds assumption showed significance, which gave us the confidence to use the ordered logistic model. If a determinant was positively associated with escalation of analgesics, absence of this variable was associated negatively with escalation or positively with de-escalation in analgesic treatment (and vice versa). This offered advantage compared with separate models for escalation and de-escalation (such as consistency of model estimates) and avoided double use of patients with a neutral development of analgesic treatment.
Results
Overall, 449 410 patients were included, of which 166 374 were in the Starter group and 283 036 in the Continuation group. The baseline characteristics of both groups are shown in table 2.
About 7.6% of all escalating patients were residing in a deprived neighbourhood, and approximately 27.6% were living in an area of the highest level of urbanisation (level 1) (table 2). The majority were women, and there were more patients showing escalation (15.4%) than de-escalation (11.3%) of analgesic treatment. The majority of patients continued a neutral analgesic treatment regime (73.3%) (table 2). Most of the patients were treated at level 2 or level 4 of analgesic potency. Almost all patients were using other medications, regardless of the different categories in table 2 (84.5%). Around half were using psychotropic medication (45.2%), most were using somatic co-medication (78.1%) and more than a third were using both (38.8%) (table 2).
The Starter group mainly initiated an analgesic at level 2 (70.9%) and level 4 (19.9), whereas only 2.6% directly initiated at level 5. However, analgesic potency levels 4 and 5 increased up to 20.5% and 5.2%, respectively by the time of the last prescription in the Starter group (table 2).
In the Continuation group, patients already received analgesics at a higher level of potency at inclusion compared with the last observed level of medication potency in the Starter group. Levels 4 and 5 were observed in 35.6% at the start of the observation period, increasing to 38.3% at the end of the observation period (table 2).
Escalation of analgesic treatment was observed more often in deprived neighbourhoods and in areas of the highest levels of urbanisation (16.8% and 16.1% in the Continuation group and 16.5% and 16.3% in the Starter group, respectively) compared with rural areas (15.1%) and non-deprived neighbourhoods (15.3.%) (table 2). The proportion of patients with neutral development of analgesic treatment was lower in deprived neighbourhoods and areas with the highest degree of urbanisation compared with less densely populated areas (table 2).
In the Starter group, escalation was positively associated with lower level of first observed pain medication (highest adjusted OR 58.23 at analgesic level 1, 95% CI 53.60 to 63.27; lowest OR 1.36 at analgesic level 4, 95% CI 1.27 to 1.45; compared with reference level 5) (table 3). Escalation was furthermore associated, in a dose–response fashion, with level of urbanisation (highest adjusted OR 1.24 at urbanisation level 1, 95% CI 1.18 to 1.30; compared to reference level 5) (table 3). Furthermore, a weak but additional association existed between escalation and neighbourhood deprivation (OR 1.07, 95% CI 1.02 to 1.11) (table 3). Use of tricyclic antidepressants (TCA), mood stabilisers (OR 1.40, 95% CI 1.10 to 1.79), sedatives, cardiovascular medication (OR 1.16, 95% CI 1.13 to 1.19) and medications for other somatic conditions was associated with analgesics escalation, when prescribed before start of analgesics (table 3). Similarly, in the Starter group, escalation of analgesic treatment was also associated with the use of selective noradrenaline–serotonin reuptake inhibitors (SNRI), sedatives (OR 1.82, 95% CI 1.74 to 1.89), TCA (OR 2.19, 95% CI 2.03 to 2.36) and antipsychotics (OR 2.42, 95% CI 2.18 to 2.67) when prescribed after start of analgesics (table 3). Negative associations with escalation (ie, positive association with de-escalation) were apparent for younger age, female sex and pharmacological migraine treatment. Furthermore, use of antipsychotics was negatively associated with escalation if started simultaneously with analgesic treatment (OR 0.69, 95% CI 0.58 to 0.83) (table 3).
The use of selective serotonin reuptake inhibitors (SSRI), before, at or after start of analgesic treatment was not associated with escalation of analgesics in the Starter group (table 3).
In the Starter group, the original fully saturated model and the model after backward elimination revealed the same variables associated significantly with escalation in chronic pharmacological pain treatment (table 3).
In the Continuation group, escalation of analgesics was positively associated with lowest levels of first observed analgesics (highest adjusted OR 16.00 at analgesic level 1, 95% CI 15.20 to 16.85; lowest OR 1.55 at analgesic level 4, 95% CI 1.50 to 1.61; compared with reference level 5) (table 4). Furthermore, escalation was associated with level of urbanisation in a dose–response fashion (highest adjusted OR 1.18 at level 1, 95% CI 1.14 to 1.23; compared with reference level 5) (table 4). There was also an association between escalation and deprived neighbourhoods, use of SSRI, SNRI, TCA, total antipsychotics and sedatives (OR 1.31, 95% CI 1.29 to 1.34) as well as use of somatic co-medication (OR 1.12, 95% CI 1.10 to 1.14) (table 4). De-escalation was associated with female sex, younger age, treatment of migraine and use of second-generation antipsychotics (OR 0.80, 95% CI 0.70 to 0.91) (table 4).
The saturated model showed that 22 of 29 variables had significant associations. All these variables remained significant in the backward elimination approach. One additional variable displayed a significant association after backward elimination: ‘TCA high dosage’. The OR's did not (16 variables) or only minimally (six variables) differ between the fully saturated model and the backward elimination model. The only variable showing a degree of difference was ‘TCA total’ (fully saturated model: OR 1.19, 95% CI 1.06 to 1.32; backward elimination model: OR 1.33, 95% CI 1.29 to 1.36) (table 4).
Over time, the escalation process continues even after the first 6 months of chronic analgesic treatment. In the Starter group, opioid analgesics (level 4/5) were dispensed in 29.8% of patients living in a deprived neighbourhood. In contrast, 45.1% of patients in deprived neighbourhoods used opioids in the Continuation group, after 1 year of prescription. A similar but attenuated development was seen at urbanisation level 1 and levels 2–5 (table 2).
Discussion
Escalation of chronic analgesic treatment was observed more often in urban areas and deprived neighbourhoods within urban areas, suggesting that pain outcomes either are associated with individual characteristics that are more prevalent in urban and deprived areas or subject to contextual influences, like area-level stress or social fragmentation, regardless of individual-level characteristics. One individual-level variable that may explain part of the association with urbanicity and deprivation is socioeconomic status,27 28 which was not available for inclusion in the model. Nevertheless, the fact that the association with urbanicity remained with deprivation adjusted for in the same model suggests that urban effects may not be reducible entirely to individual-level socioeconomic status. Furthermore, although neighbourhood deprivation and urbanicity are correlated, additional association of deprivation with escalation of analgesics exists over and above urbanisation, indicating that other parameters than population density are involved too. However, the findings could also be attributed to reverse causation, that is, patients with worsening pain may move into more urban and deprived neighbourhoods as a consequence of being disabled due to ill health.
Regardless of the underlying mechanism, results clearly echo findings of unconfounded higher rates of poor mental health in areas of higher levels of urbanisation and greater neighbourhood deprivation11 29 and suggest that the outcome of mental disorder comorbidity associated with somatic disorders may show similar predictable variation. Functional pain syndromes and psychiatric disorders show high levels of interdependency,30–35 and psychiatric conditions enhance severity of somatic symptoms.36 Thus, part of the mechanism underlying the association between pharmacological pain escalation and urban environment may be explained by urbanisation increasing the risk for mental ill health. This hypothesis is supported by the findings that, as in both the Starter and the Continuation groups, escalation of chronic analgesic treatment was associated with urban environment and neighbourhood deprivation and also with prescription of various psychotropic medications prescribed in association. In general, the positive association of escalating analgesic treatment with psychotropic medication was as strong or even stronger than the association with prescribed somatic co-medication, with the exception of the observed de-escalating effect, in the Continuation group, of second-generation antipsychotics, which possess powerful analgesic properties.37 38 This is in accordance with the literature, given the fact that psychiatric conditions can enhance symptom severity in somatic patients,36 which sometimes may impact even more than the somatic condition itself.39
Although the absolute difference between analgesic escalation in the urban versus less urban environment was small, this difference may be relevant from a public health perspective, given the high rate of painful conditions in the general population. Furthermore, prevention of persistent pain states is relevant with regard to costs.40 Given the well-known increase of healthcare costs in complex patients with frequent utilisation of healthcare, with or without psychiatric comorbidity, only a small number of patients is required to cause relevant clinical cost changes.41 A more effective treatment of persistent pain, including treatment of psychiatric comorbidity, may have a cost-saving effect. Targeting populations with painful conditions for early recognition and treatment of mental health problems may be cost-effective from a public health perspective and also represents an area of considerable unmet clinical need since opioid escalation is an inflationary development in the treatment of painful conditions.42 43 Moreover, broadening the pain agenda to a better understanding of associated mental health problems could minimise failed surgery outcomes, for example, in patients with undetected mental disorders. For instance, new surgical procedures were found to be more common in chronic back pain patients with post-traumatic stress disorder compared with chronic back pain patients without post-traumatic stress disorder.44 Similarly, depression was demonstrated in 47.4% of patients with low back pain who had no surgery, in 50% of those with one surgical procedure and in 62.5% of those who had undergone surgery more than once.45 Influencing central pain sensitisation by providing adequate antidepressant treatment in depressive conditions may help prevent surgical escalation.
ORs for escalation of analgesics in relation to original level of analgesics may represent ceiling effects in both the Starter and the Continuation groups—patients already at level 5 have nowhere stronger to go; treatment of patients at level 1 at baseline can escalate to stronger medication. Ceiling effects may reflect the pattern of prescribing analgesics in general practice. Given these, it has been suggested that the WHO analgesic ladder is in need of updating.46 For example, Vargas-Schaffer is broadening the ladder with a fourth surgical step; in the current article, however, we guide attention to treatment aspects related to underestimated mental disorder comorbidity in persistent pain states.
The speculative question remains to what degree escalation of analgesic treatment and its association with psychotropic medication reflects therapeutic paradigms to remedy pain, treatment of psychiatric comorbidity or a cause of psychopathology. For instance, in the Starter and the Continuation groups of chronic analgesic treatment, escalation of analgesics was consistently and positively associated with the use of TCA. This prescription habit may reflect routine off-label paradigms in the pharmacological treatment of pain syndromes.10 47–49 However, given the evidence regarding TCA's efficacy in pain conditions, negative rather than positive associations with escalation of analgesics should have been expected. More likely, since the association with TCAs was as strong as the association of sedatives with analgesic escalation, it may be a reflection of affective or addictive comorbidity in persistent pain, for example, in vulnerable cases of opiate-induced sensitisation, tolerance and hyperalgesia.50–56 Our data indicate that escalation of analgesics may represent an ongoing process after even months of treatment, which occurs not exclusively in the context of environmental deprivation. Since prescriptions of psychotropic and (attenuated) somatic medication show a similar pattern over time, escalation may also be driven to a degree by patient factors such as opioid tolerance, opioid-induced hyperalgesia53 or disease progression.
Given the literature on this topic,47–51 57 negative associations of particularly antidepressants with escalation of chronic analgesic treatment would have been expected. Nevertheless, negative associations between escalation of chronic analgesic treatment were also found, for example, with migraine treatment (tables 3 and 4). Moreover, the use of antipsychotics was negatively associated with analgesic escalation in the Starter group—if prescribed after start of analgesic treatment. In the Continuation group, de-escalation was specifically associated with the use of second-generation antipsychotics. This outcome is interesting and deserves further investigation, given that limited evidence for the efficacy of antipsychotics in pain conditions already exists.37 38
The results of the current study should be seen in the light of several limitations. The use of routine data rather than a targeted data collection could have caused more random error resulting in type II error. Unidentified confounding may have played a role, as randomisation was not possible and pre-design and post-design are sensitive to effects of unmeasured changes affecting outcome measures over time. Another limitation is the lack of outcomes other than urbanisation, psychotropic medication or somatic co-medication. For instance, there were no estimates regarding care consumption or illness-related sick leave. Changes in patient-related outcomes like illness severity, global functioning, quality of life and treatment satisfaction should also form part of prospective evaluations. The type of data used is subject to the possibility of ecological fallacy: people whose pharmacy is in a deprived or urban neighbourhood do not necessarily experience that level of deprivation or urbanicity. Furthermore, this study only collected data over a 12-month period. Affect and pain monitoring deserves longer evaluation. Finally, due to the study design, associations do not allow for causal inference.
What is already known on this topic.
Numerous observational studies have observed higher rates of poor mental health in urban and deprived neighbourhood environments.
Pain syndromes and mental disorders show high levels of interdependency, and mental disorders are known to enhance severity of somatic symptoms.
What this study adds.
Escalation of chronic analgesic treatment in persistent pain is associated with urban environments and deprived neighbourhoods and occurs in a context of increased psychotropic medication prescribing, suggesting that pain outcomes and mental health outcomes share factors that increase risk and remedy suffering. Early recognition of mental health problems may represent an area of unmet clincal need in persistent pain conditions
Supplementary Material
Supplementary Material
Footnotes
To cite: Leue C, Buijs S, Strik J, et al. Observational evidence that urbanisation and neighbourhood deprivation are associated with escalation in chronic pharmacological pain treatment: a longitudinal population-based study in the Netherlands. BMJ Open 2012;2:e000731. doi:10.1136/bmjopen-2011-000731
Contributors: CL, SB and JvO were principal investigators of the study. SB analysed the data in collaboration with CL, JvO and JS. CL and JvO drafted the paper. All authors contributed to subsequent drafts of the paper, including the final version. JvO is the guarantor.
Funding: Eli Lilly provided funding to IMS Health for data analysis. External funding did not support any other aspect of the study. All authors contributed independently from funders to this study, and all authors had full access to and can take responsibility for the data and analyses.
Competing interests: None.
Ethics approval: Data were anonymous, reflecting routine general practice. In the Netherlands, no ethical commission approval is required for analyses using anonymous data acquired in routine practice.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional data from the study available.
References
- 1.Lofors J, Sundquist K. Low-linking social capital as a predictor of mental disorders: a cohort study of 4.5 million Swedes. Soc Sci Med 2007;64:21–34 [DOI] [PubMed] [Google Scholar]
- 2.Thomas H, Weaver N, Patterson J, et al. Mental health and quality of residential environment. Br J Psychiatry 2007;191:500–5 [DOI] [PubMed] [Google Scholar]
- 3.Ivory VC, Collings SC, Blakely T, et al. When does neighbourhood matter? Multilevel relationships between neighbourhood social fragmentation and mental health. Soc Sci Med 2011;72:1993–2002 [DOI] [PubMed] [Google Scholar]
- 4.Lederbogen F, Kirsch P, Haddad L, et al. City living and urban upbringing affect neural stress processing in humans. Nature 2011;474:498–501 [DOI] [PubMed] [Google Scholar]
- 5.Hubbard CS, Labus JS, Bueller J, et al. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J Neurosci 2011;31:12491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murrough JW, Huang Y, Hu J, et al. Reduced amygdala serotonin transporter binding in posttraumatic stress disorder. Biol Psychiatry 2011;70:1033–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith HS, Harris R, Clauw D. Fibromyalgia: an afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician 2011;14:E217–45 [PubMed] [Google Scholar]
- 8.Price DD, Craggs JG, Zhou Q, et al. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models and neuroimaging. Neuroimage 2009;47:995–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wise TN, Fishbain DA, Holder-Perkins V. Painful physical symptoms in depression: a clinical challenge. Pain Med 2007;8(Suppl 2):S75–82 [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg DL. Pain/Depression dyad: a key to a better understanding and treatment of functional somatic syndromes. Am J Med 2010;123:675–82 [DOI] [PubMed] [Google Scholar]
- 11.Peen J, Schoevers RA, Beekman AT, et al. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr Scand 2010;121:84–93 [DOI] [PubMed] [Google Scholar]
- 12.Crump C, Sundquist K, Sundquist J, et al. Neighborhood deprivation and psychiatric medication prescription: a Swedish national multilevel study. Ann Epidemiol 2011;21:231–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netelenbos JC, Geusens PP, Ypma G, et al. Adherence and profile of non-persistence in patients treated for osteoporosis–a large-scale, long-term retrospective study in The Netherlands. Osteoporos Int 2011;22:1537–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buurma H, Bouvy ML, De Smet PA, et al. Prevalence and determinants of pharmacy shopping behaviour. J Clin Pharm Ther 2008;33:17–23 [DOI] [PubMed] [Google Scholar]
- 15.http://www.sfk.nl/nieuws-publicaties/PW/2003/2003-15.htm
- 16.World Health Organization Traitement De La Douleur Cancéreuse. Geneva, Switzerland: World Health Organization, 1987 [Google Scholar]
- 17.Wiffen PJ, Derry S, Moore RA, et al. Carbamazepine for acute and chronic pain in adults. Cochrane Database Syst Rev 2011;(1):CD005451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiffen PJ, Derry S, Moore RA, et al. Lamotrigine for acute and chronic pain. Cochrane Database Syst Rev 2011;(2):CD006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore RA, Wiffen PJ, Derry S, et al. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2011;(3):CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore RA, Straube S, Wiffen PJ, et al. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev 2009;(3):CD007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perucca E, Tomson T. The pharmacological treatment of epilepsy in adults. Lancet Neurol 2011;10:446–56 [DOI] [PubMed] [Google Scholar]
- 22.Van Os J, Hanssen M, Bijl RV, et al. Prevalence of psychotic disorder and community level of psychotic symptoms: an urban-rural comparison. Arch Gen Psychiatry 2001;58:663–8 [DOI] [PubMed] [Google Scholar]
- 23.Central Bureau of Statistics Bevolking der gemeenten Van Nederland. The Hague, The Netherlands: CBS Publications, 1993 [Google Scholar]
- 24.Wiegers TA. Herijking Stedelijke Achterstandsgebieden. Utrecht, The Netherlands: NIVEL publications, 2008 [Google Scholar]
- 25.uni 2009. http://www.naz.nl/
- 26.http://www.sas.com/
- 27.Drukker M, van Os J. Mediators of neighbourhood socioeconomic deprivation and quality of life. Soc Psychiatry Psychiatr Epidemiol 2003;38:698–706 [DOI] [PubMed] [Google Scholar]
- 28.Skapinakis P, Weich S, Lewis G, et al. Socio-economic position and common mental disorders. Longitudinal study in the general population in the UK. Br J Psychiatry 2006;189:109–17 [DOI] [PubMed] [Google Scholar]
- 29.March D, Hatch SL, Morgan C, et al. Psychosis and place. Epidemiol Rev 2008;30:84–100 [DOI] [PubMed] [Google Scholar]
- 30.Hotopf M, Mayou R, Wadsworth M, et al. Temporal relationships between physical symptoms and psychiatric disorder. Results from a national birth cohort. Br J Psychiatry 1998;173:255–61 [DOI] [PubMed] [Google Scholar]
- 31.Kroenke K, Jackson JL, Chamberlin J. Depressive and anxiety disorders in patients presenting with physical complaints: clinical predictors and outcome. Am J Med 1997;103:339–47 [DOI] [PubMed] [Google Scholar]
- 32.Barsky AJ, Orav EJ, Bates DW. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry 2005;62:903–10 [DOI] [PubMed] [Google Scholar]
- 33.Fink P, Hansen MS, Oxhoj ML. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res 2004;56:413–18 [DOI] [PubMed] [Google Scholar]
- 34.Levy RL, Olden KW, Naliboff BD, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology 2006;130:1447–58 [DOI] [PubMed] [Google Scholar]
- 35.Leue C, Driessen G, Strik JJ, et al. Managing complex patients on a medical psychiatric unit: an observational study of university hospital costs associated with medical service use, length of stay, and psychiatric intervention. J Psychosom Res 2010;68:295–302 [DOI] [PubMed] [Google Scholar]
- 36.Meyer T, Klemme H, Herrmann C. Depression but not anxiety is a significant predictor of physicians' assessments of medical status in physically ill patients. Psychother Psychosom 2000;69:147–54 [DOI] [PubMed] [Google Scholar]
- 37.Freedenfeld RN, Murray M, Fuchs PN, et al. Decreased pain and improved quality of life in fibromyalgia patients treated with olanzapine, an atypical neuroleptic. Pain Pract 2006;6:112–18 [DOI] [PubMed] [Google Scholar]
- 38.Seidel S, Aigner M, Ossege M, et al. Antipsychotics for acute and chronic pain in adults. Cochrane Database Syst Rev 2008;(4):CD004844. [DOI] [PubMed] [Google Scholar]
- 39.Kim KW, Han JW, Cho HJ, et al. Association between comorbid depression and osteoarthritis symptom severity in patients with knee osteoarthritis. J Bone Joint Surg Am 2011;93:556–63 [DOI] [PubMed] [Google Scholar]
- 40.Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain 2000;84:95–103 [DOI] [PubMed] [Google Scholar]
- 41.de Jonge P, Huyse FJ, Stiefel FC. Case and care complexity in the medically ill. Med Clin North Am 2006;90:679–92 [DOI] [PubMed] [Google Scholar]
- 42.Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA 2012;307:940–7 [DOI] [PubMed] [Google Scholar]
- 43.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011;305:1315–21 [DOI] [PubMed] [Google Scholar]
- 44.Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychopathology: research findings and theoretical considerations. Psychosom Med 2002;64:773–86 [DOI] [PubMed] [Google Scholar]
- 45.Vereckei E, Susanzky E, Kopp M, et al. Psychosocial, educational, and somatic factors in chronic non-specific low back pain. Rheumatol Int. Published Online First: 3 April 2012. doi:10.1007/s00296-012-2398-0 [DOI] [PubMed] [Google Scholar]
- 46.Vargas-Schaffer G. Is the WHO analgesic ladder still valid? Twenty-four years of experience (In English, French). Can Fam Physician 2010;56:514–17, e202–5. [PMC free article] [PubMed] [Google Scholar]
- 47.Hauser W, Bernardy K, Uceyler N, et al. Treatment of fibromyalgia syndrome with antidepressants: a meta-analysis. JAMA 2009;301:198–209 [DOI] [PubMed] [Google Scholar]
- 48.Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut 2009;58:367–78 [DOI] [PubMed] [Google Scholar]
- 49.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev 2007;(4):CD005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krebs EE, Gaynes BN, Gartlehner G, et al. Treating the physical symptoms of depression with second-generation antidepressants: a systematic review and metaanalysis. Psychosomatics 2008;49:191–8 [DOI] [PubMed] [Google Scholar]
- 51.Arnold LM, Hudson JI, Wang F, et al. Comparisons of the efficacy and safety of duloxetine for the treatment of fibromyalgia in patients with versus without major depressive disorder. Clin J Pain 2009;25:461–8 [DOI] [PubMed] [Google Scholar]
- 52.Arnold LM, Hudson JI, Keck PE, et al. Comorbidity of fibromyalgia and psychiatric disorders. J Clin Psychiatry 2006;67:1219–25 [DOI] [PubMed] [Google Scholar]
- 53.Bekhit MH. Opioid-induced hyperalgesia and tolerance. Am J Ther 2010;17:498–510 [DOI] [PubMed] [Google Scholar]
- 54.Berton O, McClung CA, Dileone RJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006;311:864–8 [DOI] [PubMed] [Google Scholar]
- 55.Upadhyay J, Maleki N, Potter J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 2010;133:2098–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006;104:570–87 [DOI] [PubMed] [Google Scholar]
- 57.Rayner L, Price A, Evans A, et al. Antidepressants for depression in physically ill people. Cochrane Database Syst Rev 2010;(3):CD007503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.