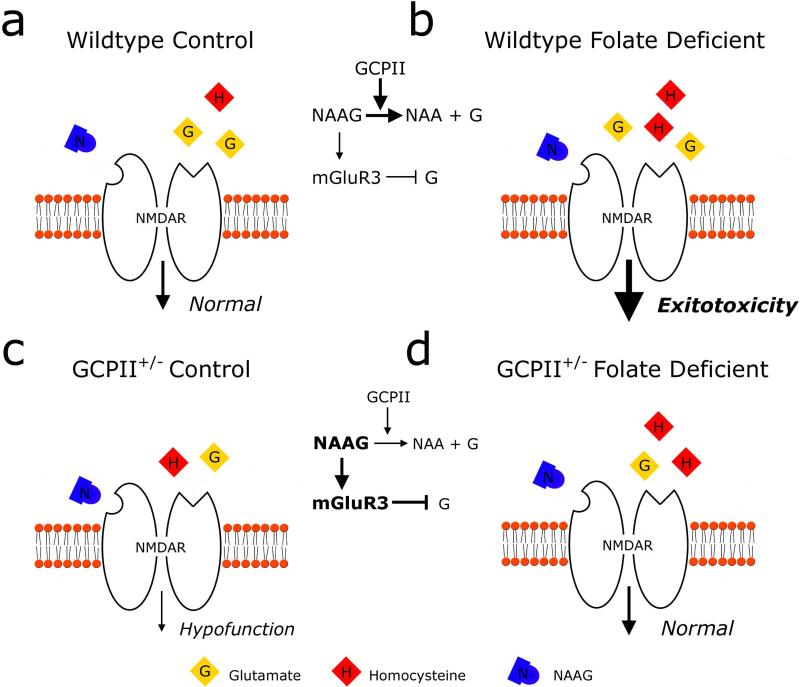
Figure 5. Proposed NMDA receptor mediated mechanism of reciprocal behavioral protection.
A model showing the relative levels of NMDAR agonists, glutamate (G) and homocysteine (H), and antagonist NAAG (N) in the synaptic cleft during glutamatergic neurotransmission in the four experimental groups. In wildtype mice on a control diet (a) or folate deficient diet (b), GCPII hydrolyzes NAAG into NAA and glutamate similarly; therefore, both groups exhibit similar levels of glutamate and NAAG. In wildtype folate deprived mice (b), increased levels of homocysteine can additionally activate NMDAR's and cause excitotoxicity. Reduced function of GCPII in heterozygous mice on both a control diet (c) and a folate deficient diet (d) increases the levels of NAAG and decreases NAA and glutamate levels. NAAG likely predominately activates presynaptic mGluR3 receptors to further inhibit glutamate release. Thus, both GCPII heterozygous groups exhibit a similar decrease in levels of glutamate compared to wildtype groups. In GCPII mice on a control diet (c), reduced glutamate results in NMDAR hypofunction whereas in GCPII folate deficient mice (d), decreased levels of glutamate are paired with increased homocysteine that results in overall normal levels of activity.